Trevor Disease (Hemimelic Epiphyseal Displasia): 12 year Follow up Case Report and Literature Review
- *Corresponding Author:
- Prof. Robinson Esteves Santos Pires
Avenida do Contorno, 9530, Barro Preto, 30110-060 Belo Horizonte, Minas Gerais, Brazil.
E-mail: robinsonestevespires@mail.com
Abstract
Trevor disease or hemimelic epiphyseal dysplasia is a rare skeletal developmental disorder characterized by asymmetric overgrowth of cartilage in the epiphyses. Histologically, it is an epiphysis osteochondroma. The symptom onset occurs primarily during childhood. Males are 3 times more affected than females. The most common symptom is a painless bony mass around the ankle or knee, followed by swelling, restricted range of motion and deformity. Imaging diagnosis is based on plain radiographs, computed tomography scans and magnetic resonance imaging. Treatment depends on the deformities, symptoms, location and amount of epiphysis involvement. Asymptomatic patients require no treatment. When no deformities are identified, simple mass excision is the treatment choice. If the mass causes epiphyses asymmetry, resection must be combined with osteotomies. The present study reports a case of Trevor disease in a female patient with 12‑year follow‑up. A general review of Trevor disease was also performed
Keywords
Ankle, Foot, Foot bones, Foot deformities, Foot diseases
Introduction
Hemimelic epiphyseal dysplasia (HED) is a rare skeletal development disorder characterized by asymmetric growth of epiphyseal cartilage. Clinically and pathologically, HED resembles epiphyseal osteochondroma and most commonly affects lower limb bones.
The condition was first described in 1926 by Mouchet and Belot, who called it tarsomegaly. However in 1950, Trevor described 10 consecutive cases and grouped them into one distinct entity, naming it tarso epiphysary aclasia.[1,2]
Due to previous unsuitable designations, Fairbank renamed Trevor disease as HED. Tarsal involvement is inconstant and shows true epiphyseal dysplasia.[3]
HED is characterized by the presence of irregular isolated ossification centers involving the epiphysis. In a few cases however, epiphysis may be totally involved. During growth, ossification centers develop individually or together, resulting in bone mass increase and asymmetrical epiphysis resembling exostoses.[4,5]
The aim of this study is to perform a general review of this rare and challenging disease. Informed consent was obtained.
Case Report
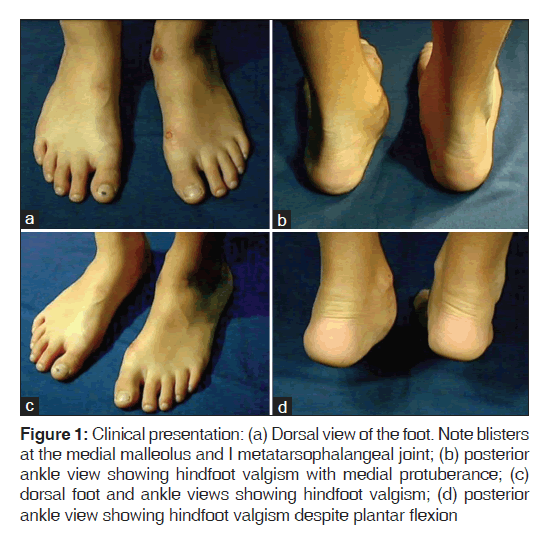
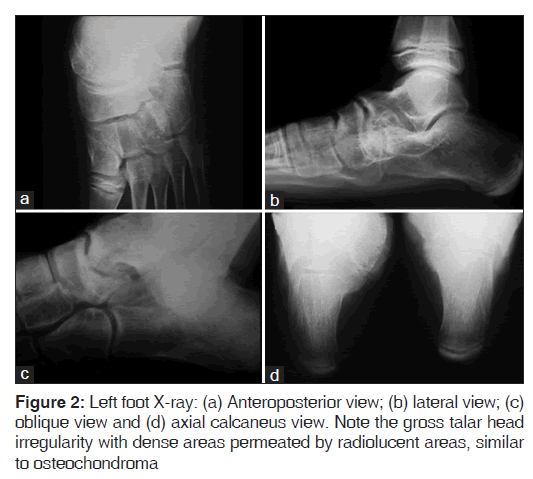
The present case report is about a 12-year-old female patient who presented medial foot and ankle pain related to recreational activities. A rigid mass on the medial side of the foot was perceived, followed by progressive functional incapacity. Due to the foot and ankle medial protuberance, local trauma was frequent; producing skin blisters [Figure 1]. Physical examination showed rigid hindfoot valgism, blisters and medial hindfoot prominences, causing pain when touched. Figure 2 shows foot and ankle X-rays.
Figure 1: Clinical presentation: (a) Dorsal view of the foot. Note blisters at the medial malleolus and I metatarsophalangeal joint; (b) posterior ankle view showing hindfoot valgism with medial protuberance; (c) dorsal foot and ankle views showing hindfoot valgism; (d) posterior ankle view showing hindfoot valgism despite plantar flexion
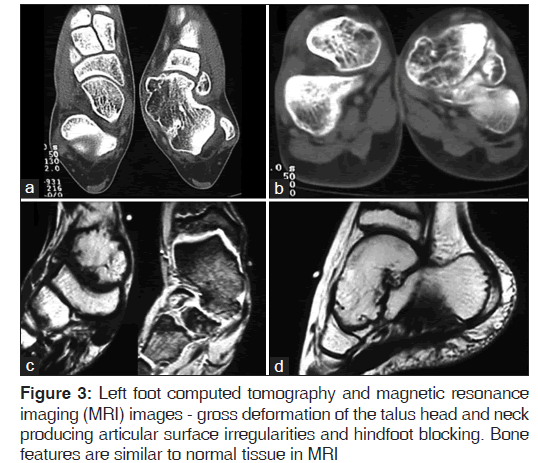
The images show a talar head mass which deforms the normal anatomy. The mass exceeds the talar head limit, occupying the sinus tarsi space [Figure 3]. The joint irregularity clearly explains inversion-eversion blocking and pain during physical activities.
Considering the symptoms and functional limitations, the patient underwent surgical procedure, receiving medial and lateral approaches.
It was impossible to remove and model the anomalous tissue due to gross deformity and articular degeneration. Therefore, talo-navicular arthrodesis was performed after ample resection of the abnormal tissue [Figure 4].
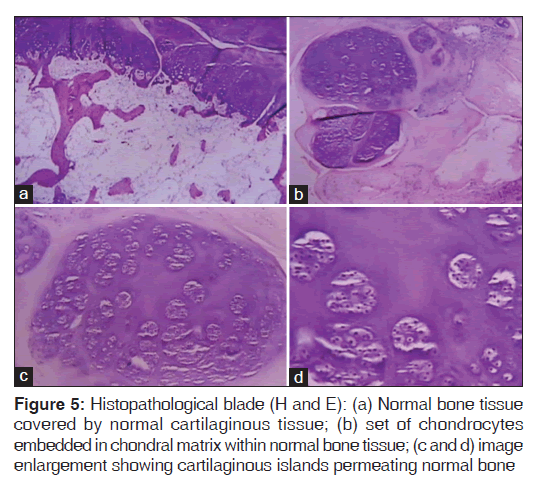
Histopathological analysis showed a tissue compatible with articular osteochondroma. Mitotic figures and cellular changes associated with malignancy transformation were inexistent. The main feature differentiating HED from classic osteochondroma is HED epiphysis involvement. Classic osteochondroma characteristically appears in the metaphysis [Figure 5].
After surgery, weight bearing was prohibited during 8 weeks, followed by physiotherapy during 3 months.
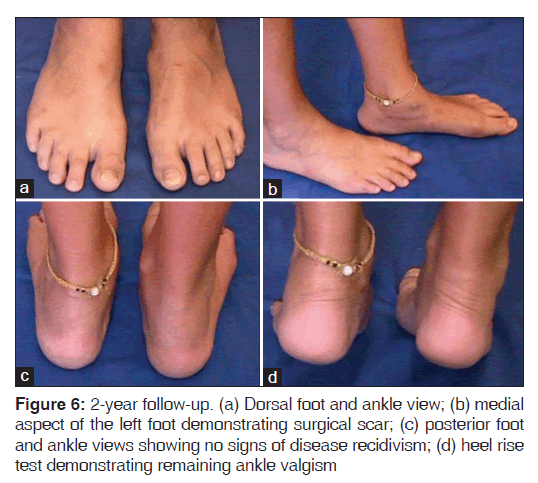
At 2 years post-surgery, the patient presented no pain, no disease recidivism and complete arthrodesis healing [Figures 6 and 7].
The patient presented good aesthetic and functional outcome despite few limitations, especially regarding sport activities.
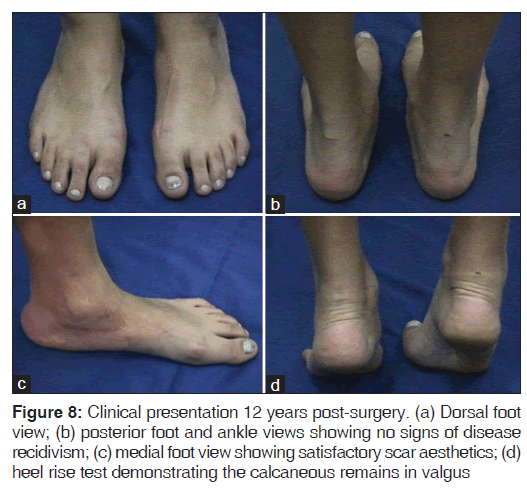
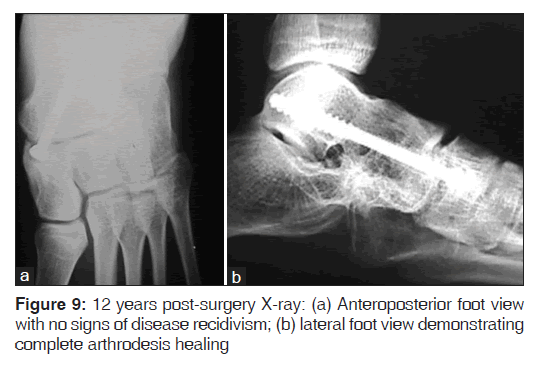
At 12 years post-surgery, when the patient was 24 years old, the last clinical and radiological evaluations showed no functional daily activity limitations and no signs of disease recidivism [Figures 8 and 9].
Although considered a successful treatment, the patient was still advised to maintain regular monitoring due to potential complications such as foot arthritis.
Discussion
HED etiology remains unknown and the authors found no references of malignant transformation in the literature.[6]
Different theories have been described to explain HED origin, but none are considered as definitive. They are:
1. Irregular cell proliferation in the superficial zone of articular cartilage[2]
2. Changes in apical development button of lower limb[3]
3. Changes in blood epiphysary vessel arrangement[2]
4. Imbalance between proliferation and cellular death mechanism[2,7]
5. Changes in fetal limb apical ectoderm, resulting in pre or post-axial disorders.[2,3]
The literature contains only one family report of HED. In 1974, Hensinger demonstrated HED in two generations of the same family.[8]
HED affects patients of all ages, however those between 2 and 14 years old are more susceptible.[5,9-11] Men are 3 times more affected than women and the incidence is 1 patient/1,000,000 live births.[11-13] The most affected bones are distal tibia and fibula (22%), talus and calcaneus (22%), distal femur (21%), proximal tibia (11%), navicular, cuboid, cuneiforms, (10%), scaphoid (2%) and scapula (1%). HED frequently involves just one limb in many places and is responsible for the hemimelic denomination. HED occurs in medial epiphysis 2 times more than lateral. Side limb predominance is irrelevant.[3,14,15]
The most frequent clinical presentation is a slow-growing, painless mass, localized at the medial side of the knee or ankle.
The mass gradually hardens and becomes painful, followed by articular stiffness and anisomely.[11,16-18] According to Fairbank findings, young patients present pain, edema and joint stiffness. Degenerative findings are more common in older patients.[3]
Deformities can develop with disease progression, depending on the affected epiphysis segment. Foot equinism and ankle and knee angular deformities are frequent.[5] At the epiphysis closure, the disease stops progression.
HED presents three classical clinical types:[15]
1. Localized: One epiphysis is affected
2. Classic: More than one epiphysis is affected in one limb (the most common presentation)
3. Generalized: The entire limb is affected (e.g.: From the pelvis to the foot).
Identifying HED without more specific exams is difficult due to several epiphyseal diseases presenting the same radiographic findings. Irregular and multicentric epiphysis opacities resemble osteochondroma.[5,11] With growing asymetric apophysis, enlargement appears and multicentric calcifications coalesce. Ossification bone cores then become confluent and mingle with normal bone tissue.[19]
Computed tomography-scan is an excellent exam to define the limit between pathological and normal bone tissues. It is also highly important in surgical planning.[5,11,14,20]
Magnetic resonance imaging safely determines the degree of soft tissues and epiphysis involvement. It is an important tool in the differential diagnosis concerning other tumoral diseases.[21]
Cintilography presents nonspecific findings, but can identify several HED affected loci.
Histologically, HED presents a normal bone mass covered by abundant cartilaginous foci of endochondral ossification, resembling osteochondroma.[3,5]
Anomalies that produce multiple epiphyseal changes such as punctate epiphyseal dysplasia, achondroplasia and aseptic necrosis are part of the differential diagnosis. However, articular calcifying tumors such as osteochondroma and carpotarsal dominant osteochondromatosis are diseases that most resemble HED.[22,23] Tarsal coalition is another differential diagnosis.
HED treatment is still controversial. It depends on the location, evolvement intensity and functional incapacity degree.
Asymptomatic patients are just periodically followed, since malignization risk is nonexistent.[11]
Surgical procedure is indicated in cases of pain, articular deformity or incongruence, or motion limitation.
A study done by Acquaviva et al. classified the injuries as extra or intra-articular. A simple excision of extra-articular lesions evolve more favorably than lesions involving articular surface.[12] Kuo et al. identified poor outcomes in tumors involving articular surface. In such patients, arthrosis was a frequent complication.[11]
Identifying and completely removing the lesion is essential to prevent tumor recidivism. Therefore, following the patient until complete skeletal maturity is mandatory, independent of anatomopathological type.[11]
Conclusion
Trevor disease is a rare skeletal developmental disorder characterized by asymmetric overgrowth of cartilage in the epiphyses. There is a lack in the literature concerning the “gold standard” for Trevor disease treatment.
We present a general review of this challenging issue, dealing important topics to help the orthopedic surgeon on the Trevor disease management.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Mouchet A, Belot J. La tarsomegalie. J Radiol Electrol 1926;10:289-93.
- Trevor D. Tarso-epiphysial aclasis; a congenital error of epiphysial development. J Bone Joint Surg Br 1950;32-B: 204-13.
- Fairbank TJ. Dysplasia epiphysialis hemimelica (tarso-ephiphysial aclasis). J Bone Joint Surg Br 1956;38-B: 237-57.
- Carlson DH, Wilkinson RH. Variability of unilateral epiphyseal dysplasia (dysplasia epiphysealis hemimelica). Radiology 1979;133:369-73.
- Kettelkamp DB, Campbell CJ, Bonfiglio M. Dysplasia epiphysealis hemimelica. A report of fifteen cases and a review of the literature. J Bone Joint Surg Am 1966;48:746-65.
- Bhosale SK, Dholakia DB, Sheth BA, Srivastava SK. Dysplasia epiphysealis hemimelica of the talus: Two case reports. J Orthop Surg (Hong Kong) 2005;13:79-82.
- Connor JM, Horan FT, Beighton P. Dysplasia epiphysialis hemimelica. A clinical and genetic study. J Bone Joint Surg Br 1983;65:350-4.
- Hensinger RN, Cowell HR, Ramsey PL, Leopold RG. Familial dysplasia epiphysealis hemimelica, associated with chondromas and osteochondromas. Report of a kindred with variable presentations. J Bone Joint Surg Am 1974;56:1513-6.
- Shinozaki T, Ohfuchi T, sWatanabe H, Aoki J, Fukuda T, Takagishi K. Dysplasia epiphysealis hemimelica of the proximal tibia showing epiphyseal osteochondroma in an adult. Clin Imaging 1999;23:168-71.
- DeVine JH, Rooney RC, Carpenter C, Pitcher JD. Dysplasia epiphysealis hemimelica in an elderly patient. Am J Orthop (Belle Mead NJ) 1997;26:223-5.
- Kuo RS, Bellemore MC, Monsell FP, Frawley K, Kozlowski K. Dysplasia epiphysealis hemimelica: Clinical features and management. J Pediatr Orthop 1998;18:543-8.
- Acquaviva A, Municchi G, Marconcini S, Mazzarella F, Occhini R, Toti P, et al. Dysplasia epiphysealis hemimelica in a young girl: Role of MRI in the diagnosis and follow-up. Joint Bone Spine 2005;72:183-6.
- Wynne-Davies R, Hall CM, Apley AG. Atlas of Skeletal Dysplasias. Edinburgh: Churchill-Livingstone; 1985.
- Gerscovich EO, Greenspan A. Computed tomography in the diagnosis of dysplasia epiphysealis hemimelica. Can Assoc Radiol J 1989;40:313-5.
- Azouz EM, Slomic AM, Marton D, Rigault P, Finidori G. The variable manifestations of dysplasia epiphysealis hemimelica. Pediatr Radiol 1985;15:44-9.
- Araujo CR Jr, Montandon S, Montandon C, Teixeira KI, Moraes FB, Moreira MA. Best cases from the AFIP: Dysplasia epiphysealis hemimelica of the patella. Radiographics 2006;26:581-6.
- Rosero VM, Kiss S, Terebessy T, Köllö K, Szöke G. Dysplasia epiphysealis hemimelica (Trevor’s disease): 7 of our own cases and a review of the literature. Acta Orthop 2007;78:856-61.
- Keret D, Spatz DK, Caro PA, Mason DE. Dysplasia epiphysealis hemimelica: Diagnosis and treatment. J Pediatr Orthop 1992;12:365-72.
- Silverman FN. Dysplasia epiphysealis hemimelica. Semin Roentgenol 1989;24:246-58.
- Wenger DR, Adamczyk MJ. Evaluation, imaging, histology and operative treatment for dysplasia epiphysealis hemimelica (Trevor disease) of the acetabulum: A case report and review. Iowa Orthop J 2005;25:60-5.
- Iwasawa T, Aida N, Kobayashi N, Nishimura G. MRI findings of dysplasia epiphysealis hemimelica. Pediatr Radiol 1996;26:65-7.
- Teixeira AB, Sá de Camargo Etchebehere EC, Santos AO, Lima MC, Ramos CD, Camargo EE. Scintigraphic findings of dysplasia epiphysealis hemimelica: A case report. Clin Nucl Med 2001;26:162.
- Maroteaux P, Le Merrer M, Bensahel H, Freisinger P. Dominant carpotarsal osteochondromatosis. J Med Genet 1993;30:704-6.






 The Annals of Medical and Health Sciences Research is a bi-monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a bi-monthly multidisciplinary medical journal.