A Molecular Insight into the Role of Inflammation in the Behavior and Pathogenesis of Odontogenic Cysts
- *Corresponding Author:
- Dr. Harkanwal Preet Singh
Department of Oral Pathology, Dasmesh Institute of Research and Dental Sciences, Faridkot, Punjab, India
E-mail: hkps0320@gmail.com
Abstract
Background: Although, odontogenickeratocyst (OKC) and dentigerous cyst (DC) are considered asdevelopmental cysts inflammation has been seen in its connective tissue wall. Inflammation is seen to alter the epithelial lining of both OKC and DC butwhether it plays in altering the behavior of these cysts is not fully understood. Aim: The present study is conducted with the help of molecular marker proliferating cellular nuclear antigen (PCNA) to assess the proliferative activity of OKC and DC and to further evaluate and correlate the effect of inflammation on the proliferative activity and hence on biological behavior of these cysts. Materials and Methods: Immunohistochemicalstaining was performed using anti‑PCNA antibody in 10 cases each of classical OKC, inflamed OKC, classical DC, and inflamed DC. The resulting data was tabulated on Microsoft excel and subjected to statistical analysis using two‑way analysis of variance test, t test and post-hoc test followed by Bonferroni test with the application of statistical package for social sciences (SPSS) (SPSS, Chicago, IL, USA) and Epi‑Info 6.04 d, Atlanta, Georgia (USA). Results: Total mean PCNA expression is statistically higher in inflamed OKC than classical OKC and inflamed DC also showed significantly higher PCNA expression than classical DC (P < 0.001). Correlation between inflammation and PCNA expression was not statistically significant (P ≥ 0.05). Conclusion: Inflammation is responsible for change in behavior of neoplastic epithelium of OKC, whereas in dentigerous it is responsible for changes in epithelial lining and hence should be treated meticulously.
Keywords
Cyst pathogenesis, Dentigerouscyst, Odontogeniccyst, Odontogenickeratocyst, Proliferatingcellular nuclear antigen
Introduction
Odontogenickeratocyst (OKC) is a clinicopathologically distinct form of odontogenic cyst known for its pathognomonic features, i.e., aggressiveness and high rates of recurrence. Recently the World Health Organization (WHO) working group on odontogenic tumors recommended the term keratocystic odontogenic tumorfor these lesions to address their neoplastic nature, which indicated that the OKC epithelial lining may have some intrinsic growth potential.[1,2]
Transformation of the keratinized epithelial lining to non-keratinized squamous epithelium is commonin OKC with inflammation, whereas inflammatory dentigerous cysts (DC) are seen to be lined predominantly or entirely by non-keratinized stratified squamous epithelium of variable thickness, sometimes with anastomosing rete ridges and odontogenic epithelial islands in sub-epithelial connective tissue.[3,4] Inflamed OKC, DC, and radicular cyst (RC) showed similar histologic picture with non-keratinized, hyperplastic epithelial lining. Such similarities in the appearance of odontogenic cyst linings (RC, DC, and OKC) associated with inflamed areas would pose a diagnostic problem for OKC as it may be mistaken for DCs. Such misdiagnosis should be avoided since the biological activities of OKC differ from those of RC or DCs.[5]
Several recent studies have found a direct influence of the inflammation on epithelial cells, either through direct adhesion of the inflammatory cells, or through an indirect response to a series of chemokines produced by inflammatory cells.[4] Over the years, many antibodies have been raised to the gene products or the relevant proteins associated with cell cycle, e.g., amongst those monoclonal antibodies proliferating cellular nuclear antigen (PCNA) and Ki-67 are probably the most widely applied.[6] PCNA expression may be used as a marker of cell proliferation because cell remains for a longer time in G1/S phase during proliferation.[7] AsPCNA is one of the several markers of cellular proliferation, high-PCNA activity is indicative of higher proliferative activity.[5,6,8] Nonetheless, the effect of inflammation on the epithelium of odontogenic cysts remains a subject of controversy, with contradictory results being portrayed.[4,6] Hence, the present study is aimed to evaluate the influence of inflammation on immunohistochemical (IHC) expression of PCNA in order to determine its bearing on behavior of odontogenic cysts.
Materials and Methods
It was a retrospective study and was approved by the institution ethics committee. Forty formalin-fixed paraffin-embedded tissue specimens of histopathologically confirmed cases of 20 parakeratotic type OKCs (10 inflamed and 10 classical) and 20 DCs (10 inflamed and 10 classical) were retrieved from the archives of oral and Maxillofacial Pathology Department of ITS Dental College, Ghaziabad (India). Diagnoses were based on the histopathologic criteria given by WHO in 1992.[9] Two set of 4 μm sections were taken and one set of sections were stained by Harris hematoxylin and eosin for histologic diagnosis while other set was stained for PCNA using IHC method.
The inflammatory density score was determined by counting inflammatory cells adjacent to basement membrane, to a depth corresponding with one histopathological field (HPF) fewer than × 400 magnifications. It was recorded in 10 separate fields and inflammatory density score for each case was calculated as the average of all HPFs examined. Inflammatory density was graded on a 4-grade scale. Grade 0: No inflammation, grade 1: < 15 cells/field, grade 2: 15-50 cells/ field, and grade 3: > 50 cells/field. Data from all individual fields were tabulated and analyzed by grouping together data from all individual fields. This approach was preferred because often there are focal variations in the epithelial lining as well as in the inflammatory infiltrate density within each cyst and proliferation may not be equally distributed along the lining. Therefore, analysis of data; from each individual field is potentially more sensitive in detection of relationships between inflammation, epithelial morphology and expression of proliferation markers. The positively stained PCNA areas showed uptake of brown color. PCNA positive cells were counted in 100 cells of each sample: 50 cells in the basal layer and 50 cells in suprabasal layer. Ten representative fields at × 400 magnifications were selected and cells were counted in each of the mentioned layers. Thus, by selecting 10 random areas, which were not in continuum with each other, a possible error in recounting the same cell was minimized.
Statistical analysis
The resulting data was analyzed using Statistical Package for Social Sciences (SPSS) (SPSS, Chicago, IL, USA) and Epi-Info 6.04 d, Atlanta, Georgia (USA). Software and Difference between the mean of two independent groups was observed by test if data is normally (evenly) distributed. Differences between the different variables were analyzed using two-way analysis of variance test and post-hoc test followed by Bonferroni test. P value < 0.05 was considered to be significant.
Results
The study consisted of 20 cases of OKC and 20 cases of DC. Two cases of OKC and nine cases of DCs showed mild inflammation (grade 1). Under moderate inflammation (grade 2), 12 cases of OKC and 5 cases of DC were there. Six cases of OKC and DC each showed severe inflammation (grade 3) [Table 1].
| Grades of inflammation | Od onto genickeratocyst (n=20) | Dentigerouscyst (n=20) |
|---|---|---|
| No inflammation (grade 0) | 0 | 0 |
| Mild inflammation (grade 1) | 2 | 9 |
| Moderate inflammation | 12 | 5 |
| (grade 2) | ||
| Severe inflammation | 6 | 6 |
| (grade 3) |
Table 1: Distribution of cases depending upon grades of inflammation
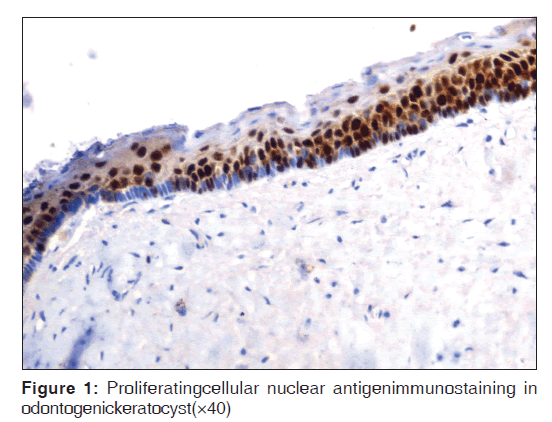
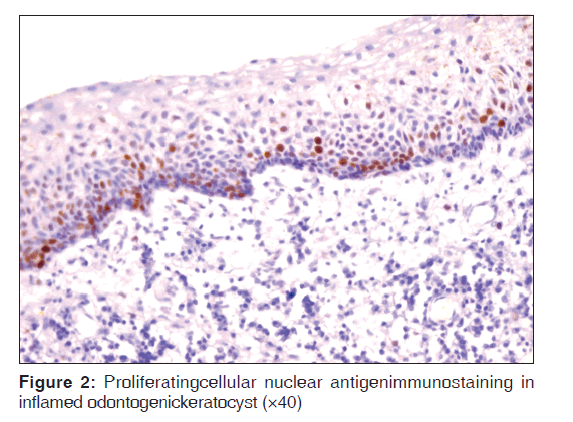
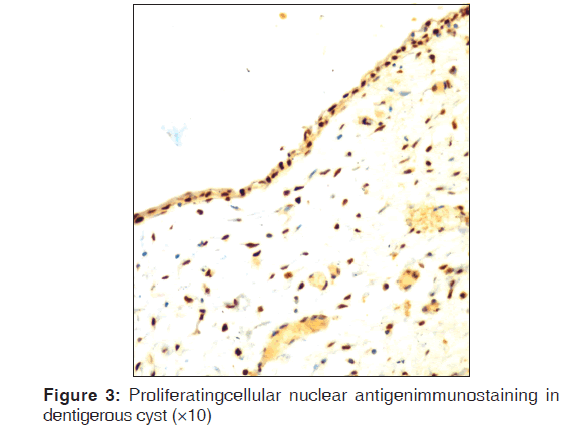
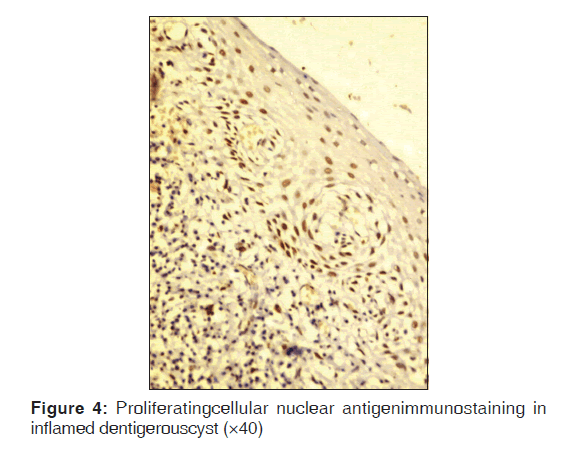
Table 2 represents the quantitative assessment of PCNA expression in basal and suprabasal cells of classical OKC [Figure 1] and inflamed OK [Figure 2], classical DC [Figure 3] and inflamed DC [Figure 4]. It revealed significantly higher expression of PCNA in suprabasal layers than basal cells of OKC, whereas, DC showed higher expression in basal layer than suprabasal layer but it was statistically non-significant. Multiple comparisons between four groups were carried using multiple Bonferroni test, which revealed that mean PCNA expression in cases of inflamed OKC and inflamed DC increased significantly as compared to its classical types [Tables 2 and 3]. Karl Pearson correlation test was applied, which showed that correlation between inflammation and total PCNA expression in all four groups were non-significant except in classical OKC [Table 4].
| Cyst | Layer | Mean (SD) | Two way ANOVA(P value) |
|---|---|---|---|
| OKC | Suprabasal | 219 (45.8) | P<0.001 |
| Basal | 63.20 (19.2) | (highly significant) | |
| Inflamed OKC | Suprabasal | 279.8 (34.86) | P<0.001 |
| Basal | 125.1 (60.53) | (highly significant) | |
| Dentigerous cyst | Suprabasal | 108.1 (31.78) | P<0.001 |
| Basal | 133.1 (30.91) | (highly significant) | |
| Inflamed | Suprabasal | 167.3 (34.30) | P<0.001 |
| dentigerous cyst | Basal | 221.4 (57.09) | (highly significant) |
OKC: Odontogenickeratocyst, ANOVA: Analysis of variance
Table 2: Quantitative assessment of proliferating cellular nuclear antigen expression in basal and suprabasal of classical and inflamed odontogenickeratocysts, classical and inflamed dentigerous cysts
| Dependentvariable | I and Jgroup | Meandifference (I-J) | P value |
|---|---|---|---|
| Suprabasal | OKC and | −60.80 | <0.01 |
| cells | I-OKC | significant | |
| DC and | −59.20 | <0.01 | |
| I-DC | significant | ||
| OKC and | 110.9 | <0.001 | |
| DC | highly significant | ||
| I-OKC and | 112.5 | <0.001 | |
| I-DC | highly significant | ||
| Basal cells | OKC and | −61.90 | 0.03 |
| I-OKC | significant | ||
| DC and | −88.30 | <0.001 | |
| I-DC | highly significant | ||
| OKC and | −69.90 | <0.01 | |
| DC | significant | ||
| I-OKC and | −96.30 | <0.001 | |
| I-DC | highly significant |
DC: Dentigerous cyst, OKC: Odontogenickeratocyst
Table 3: Multiple comparisons between four groups
| Lesion | P value | Pearson correlationco efficient (r) |
|---|---|---|
| Odontogenic | 0.04 | 0.64 |
| keratocyst | (significant) | |
| Inflamed odontogenic | 0.48 | 0.25 |
| keratocyst | (non-significant) | |
| Dentigerous cyst | 0.34 | 0.33 |
| (non-significant) | ||
| Inflamed dentigerous | 0.05 | 0.62 |
| cyst | (non-significant) |
Table 4: Correlation between inflammation and total proliferating cellular nuclear antigen expression in inflamed odontogenickeratocyst, inflamed dentigerous cyst
Discussion
Although, OKC and DC are classified as developmental lesions, inflammation is found in the connective tissue wall in the majority of cases. In present study, OKC and DC showed varying degree of inflammation from mild to severe. Several authors stated that inflammation in the connective tissue stroma of the OKC and DC is seen in majority of cases,[5,10-12] but in contrary to this Kolár, et al.[13] observed that fibrous cyst wall usually lacks inflammatory cell infiltrate. The presence of inflammation in OKC observed in our cases, may be partly due to possible communications with the oral mucosa via perforations of the cortical bone, which have been documented in up to 39% of OKC or it may also be introduced via the periodontal ligament in cases located close to adjacent teeth.[4] To explain the inflammation in DC, Benn and Altini [3] has proposedthreefeasible mechanisms, which includes secondarily inflamed dental follicle from non-vital tooth, eruption of permanent successor into RC associated with non-vital deciduous toot hand periapical inflammation from non-vital deciduous tooth. Moure et al.[12] hypothesized that if tooth eruption is competent of generating an inflammatory process, pro-mitotic chemotactic inflammatory factors present in the connective tissue may arrive at the reduced enamel epithelium in dental follicles, whose growth factor receptors are situated in the membrane and cytoplasm giving rise to DC.
PCNA had been used in past in an attempt to measure the proliferative activity of cystic epithelium in different odontogenic cysts.[4,6,7] In the present study, mean PCNA expression in suprabasal cell layers were significantly higher than basal cell layer of OKC and infected OKC, which is also reported in previous studies.[4-8] This suggests that the highest proliferative activity is in the suprabasal cell layers. de Oliveira et al.[7] believes that OKC has proliferation and maturation patterns different from other lesions. Li, et al.[14] hypothesized that this unusual proliferation represents an epithelial disorganization and signify an evidence of epithelial dysplasia. In dysplastic lesions, in fact, increasing cellular atypia correlated well to elevated proliferative activity. This could also reflect a unique epithelial differentiation process, in which the basal cells assume some characteristics of preameloblasts, which indicate that it might have entered to some extent towards ameloblast differentiation.[14] Browne et al.[15] supported this hypothesis by stating that a cell entering to a differentiation pathway, it must first leave the cell cycle. The presence of differentiated cell in the basal layer probably accounts for the fact that the major proliferation compartment is suprabasally. This hypothesis has been justified by Matthews et al.[16] by analyzing expression of cytokeratin in which it was found that basal cell in addition to keratin 19, also contains secondary keratin 13/16 which is the marker for differentiating cells. This peculiar distribution of PCNA + cells in a suprabasal location in OKC could be due, perhaps, to inductive influences of the underlying connective tissue as suggested by Piattelli et al.[8]
Inflamed OKC showed statistically highly significant increase in PCNA positive cells in both basal and suprabasal cell layers as compared to classical OKC, which is in agreement with the previous studies[6] but Kaplan and Hirshberg [4] reported that PCNA labeling index did not significantly change between areas with low- and high-inflammation. Increased expression of PCNA is suggestive of greater proliferative activity in the epithelial cells of inflamed OKCs, which could be associated with the disruption of the typical structure of OKC linings. Inflammation has a puzzling effect on the epithelium of OKC and remains a subject of controversy, with contradictory results being portrayed.[4,5] However, we suggest that inflammation definitely play role in enhancing the aggressive behavior of OKC by increasing its proliferative potential. So, it requires more aggressive treatment protocol than classical OKC requires.
Although, there is higher PCNA expression in basal layers than suprabasal layer but this increase is not statistically significant in DC, whereas inflamed DCs showed statistically significant difference, which is in accordance with previous studies using different proliferative markers.[5,8,17-19] This suggests that the PCNA reactivity may be related to the regular maintenance of 2-3 cell layer thickness of epithelium.[7,20] Thus, it advocated the mechanism of expansion of the DC that occurs passively by accumulation of fluid in the lumen rather than epithelial proliferation.
Inflamed DC showed significantly higher PCNA expression in both basal and suprabasal layer cells as compare to DC. Martins et al.[21] also obtained similar results but using different proliferative marker Ki-67. Studies have demonstrated that growth factors and cytokines (interleukin 1, interleukin 6, and tumor necrosis factor) are released during inflammatory events.[7] Inflammatory stimuli increase cell proliferation and inflammatory cytokines may also cause cell stress. The initial stimulus for the formation of DCs has not been clearly identified, but an inflammatory infiltrate in the cystic capsule has often been described. Similarly to what happens in RCs, the inflammatory stimulus may induce epithelial cells to initiate proliferation.[7] Therefore, we interpret our results as responses to inflammatory stimuli that may be the result of the eruptive process, may make cells proliferate, but may be inconstant and present for only short periods of time. This may explain the lower PCNA percentage found in DCs in this study. Their rate of proliferation is slower than that of OKC and the expression of markers may be more closely associated with cell stress caused by the inflammatory stimulus that lead to the formation of the cyst cavity. This cavity, once formed, initiates a feedback process due to the physiology of the epithelial tissue, which proliferates in the basal layer and desquamates into the cavity. The cyst becomes denser and thus attracts fluids to maintain its osmotic balance. Therefore, once the process initiates, it becomes independent of new inflammatory stimuli.[7] So, we suggest that inflammation does not alter the behavior of DC but it is responsible for changes in epithelial lining and should be treated meticulously.
OKC showed significantly higher expression of PCNA as compared to DC which is consistent with previous studies using mitotic counting, tritiated thymidine incorporation, PCNA, Ki-67 stainingmethods[13,14,16] and hence justifies its aggressive behavior. There was statistically non-significant positive correlation between inflammatory density and PCNA value in inflamed OKC and inflamed DC. From these results it can be interpreted that there is non-uniform increase in PCNA expression with the increasing grade of inflammation. As inflammation has only secondary effect on the cystic lining, it would be more imperative that how the histogenetic precursors of these developmental cysts responds to such inflammatory stimuli. So, it can be presumed that these histogenetic precursors respond differently to inflammation. Due to the complex multivariantpathogenetic apparatus involved in OKC and DC the subjected inflammatory effect on cystic lining and behavior are inherently compound. At the histogenetic front the timming/stage and the odontogenic apparatus from which the cyst is derived from, plays a crucial role in enforcing the behavior and histological diversity of the cyst. The role of inflammation and the sub-population of this inflammatory infiltrate could both be dependent and crucial for the plausible mechanism by which these apparatus would respond. Thus, the comparison of the proliferation values and inflammatory cell populations between inflamed OKC and inflamed DC become imperative for thorough understanding of inflammatory potentials on growth of developmental odontogenic cysts. Present study also leads us to probably understand the molecular pathways underlining the role of inflammation in the progression and behavioral changes of cyst. Antigenic intercepts causing lymphocytic activation are triggered from the cells of the epithelial lining. These antigenic targets put forth the recognition of the subjacent lymphocytes, which in turn through the mediation of its chemical messengers ignites a cascade of proliferative activity, which are primarily initiated in the basal layer. Inflammation and the subpopulation of this inflammatory infiltrate could both be dependent and crucial for the plausible mechanism by which these odontogenic apparatus would respond.
Conclusion
Inflammation has puzzling effect on the cystic lining, so it would be more imperative that how the histogenetic precursors of these developmental cysts responds to such inflammatory stimuli. Inflammation is responsible for change in behavior of neoplastic epithelium of OKC, whereas in dentigeous it is responsible for changes in epithelial lining. To elucidate the exact pathways of the cysts behavior the primary focus as in present study of PCNA could be enhanced by application of molecular bioassays of cell turn over and cell proliferation kinetic studies.
Acknowledgments
Authors would like to thanks all the staff members of ITS Dental College, Ghaziabad who have contributed significantly toward this study by their rich knowledge and experience. This study is approved by Chaudhary Charan Singh University, Meerut, Uttar Pradesh, India.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Shetty DC, Urs AB, Godhi S, Gupta S. Classifying odontogenickeratocysts as benign cystic neoplasms: A molecular insight into its aggressiveness. J Maxillofac Oral Surg 2010;9:30-4.
- Philipsen HP. Keratocysticodontogenictumour. In: Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Head and Neck Tumours. Pathology and Genetics. WHO Classification of Tumours. Lyon: IARC Press; 2005. p. 306-7.
- Benn A, Altini M. Dentigerous cysts of inflammatory origin. A clinicopathologic study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1996;81:203-9.
- Kaplan I, Hirshberg A. The correlation between epithelial cell proliferation and inflammation in odontogenickeratocyst. Oral Oncol 2004;40:985-91.
- Sudiono J, Zain RB. PCNA expression in epithelial linings of odontogenic cysts. Annal Dent Univ Malaya 2003;10:1-5.
- de Paula AM, Carvalhais JN, Domingues MG, Barreto DC, Mesquita RA. Cell proliferation markers in the odontogenickeratocyst: Effect of inflammation. J Oral Pathol Med 2000;29:477-82.
- de Oliveira MG, Lauxen Ida S, Chaves AC, Rados PV, Sant’AnaFilho M. Immunohistochemical analysis of the patterns of p53 and PCNA expression in odontogenic cystic lesions. Med Oral Patol Oral Cir Bucal 2008;13:E275-80.
- Piattelli A, Fioroni M, Santinelli A, Rubini C. Expression of proliferating cell nuclear antigen in ameloblastomas and odontogenic cysts. Oral Oncol 1998;34:408-12.
- Kramer IR, Pindborg JJ, Shear M. Epithelial Jaw Cyst. Histological typing of odontogenic Tumours. 2nd ed. Berlin: Springer-Verlag; 1992. p. 35-6.
- Vij R, Vij H, Rao NN. Evaluation of collagen in connective tissue walls of odontogenic cysts: A histochemical study. J Oral Pathol Med 2011;40:257-62.
- Hirshberg A, Sherman S, Buchner A, Dayan D. Collagen fibres in the wall of odontogenickeratocysts: A study with picrosirius red and polarizing microscopy. J Oral Pathol Med 1999;28:410-2.
- Moure SP, Carrard VC, Lauxen IS, Manso PP, Oliveira MG, Martins MD, et al. Collagen and elastic fibers in odontogenic entities: Analysis using light and confocal laser microscopic methods. Open Dent J 2011;5:116-21.
- Kolár Z, Geierová M, Bouchal J, Pazdera J, Zboril V, Tvrdý P. Immunohistochemical analysis of the biological potential of odontogenickeratocysts. J Oral Pathol Med 2006;35:75-80.
- Li TJ, Browne RM, Matthews JB. Quantification of PCNA + cells within odontogenic jaw cyst epithelium. J Oral Pathol Med 1994;23:184-9.
- Browne RM. Per[cyst] ent growth: The odontogenickeratocyst 40 years on. Ann R Coll Surg Engl 1996;78:426-33.
- Matthews JB, Mason GI, Browne RM. Epithelial cell markers and proliferating cells in odontogenic jaw cysts. J Pathol 1988;156:283-90.
- Kimi K, Kumamoto H, Ooya K, Motegi K. Immunohistochemical analysis of cell-cycle- and apoptosis-related factors in lining epithelium of odontogenickeratocysts. J Oral Pathol Med 2001;30:434-42.
- Tosios KI, Kakarantza-Angelopoulou E, Kapranos N. Immunohistochemical study of bcl-2 protein, Ki-67 antigen and p53 protein in epithelium of glandular odontogenic cysts and dentigerous cysts. J Oral Pathol Med 2000;29:139-44.
- Edamatsu M, Kumamoto H, Ooya K, Echigo S. Apoptosis-related factors in the epithelial components of dental follicles and dentigerous cysts associated with impacted third molars of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005;99:17-23.
- Gadbail AR, Chaudhary M, Patil S, Gawande M. Actual proliferating index and p53 protein expression as prognostic marker in odontogenic cysts. Oral Dis 2009;15:490-8.
- Martins CA, Rivero ER, Dufloth RM, Figueiredo CP, Vieira DS. Immunohistochemical detection of factors related to cellular proliferation and apoptosis in radicular and dentigerous cysts. J Endod 2011;37:36-9.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.