A Review on Polycystic Kidney Disease
2 Department of Urology and the Kidney Transplant Unit, Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Sour, Srinagar, Jammu and Kashmir, India
Received: 29-Sep-2022, Manuscript No. AMHSR-22-76182; Editor assigned: 03-Oct-2022, Pre QC No. AMHSR-22-76182 (PQ); Reviewed: 17-Oct-2022 QC No. AMHSR-22-76182; Revised: 30-Jan-2023, Manuscript No. AMHSR-22-76182 (R); Published: 06-Feb-2023, DOI: 10.54608.annalsmedical.2023.83
Citation: Hassan AU, et al. A Review on Polycystic Kidney Disease. Ann Med Health Sci Res. 2023;13:439-441
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: Polycystic kidney diseases are common disorders and are considered as common causes of end stage renal disease, both in children and in adults. General surgeons, neonatologists, pediatric urologists, nephrologists and general physicians frequently encounter patients with polycystic kidney diseases. The article describes the main embryological reasons behind cyst formation in polycystic disease not restricted to kidneys only but to other organs as well.
Method: The study was compiled in SKIMS Soura and SKIMS Bemina with review of literature from all standard text, latest references from standard indexed journals along with determining the basic embryological basis of this disease.
Conclusion: It is prudent for a physician, surgeon, nephrologist or urologist to completely understand the embryological basis the defects in cilia, polycystins and cellular signaling behind the polycystic kidney disease reducing progression to kidney failure requiring dialysis and transplantation.
Keywords
Polycystic; PKD; Polycystins; Cellular; Signaling; Genes; Adhesion; Matrix; Proteins; Heterotrimeric
Introduction
Kidneys develop from mesoderm. The nephrogenic cord starts developing at fourth week along with development of pronephros, mesonephric and metaphors and development is complete by 36th week of gestation [1]. Normal interaction between embryonic tissue, mesonephric duct, ureteric bud and metanephric blastema is critical for normal development of urinary tract especially kidneys. Agenesis can occur in absence of this fusion. Abnormal interaction between, ureteric bud and mesonephric blastema leads to abnormal fusion and development of cysts which has got profound effects as the cysts replace the normal functioning renal tissue [2]. ADPKD is a common disease that mostly presents in adults, whereas ARPKD is a rarer and often more severe form of Polycystic Kidney Disease (PKD) that usually presents in neonatal period. Though following different patterns of inheritance, the pathogenesis of ADPKD and ARPKD reveals similar causation between them.
Literature Review
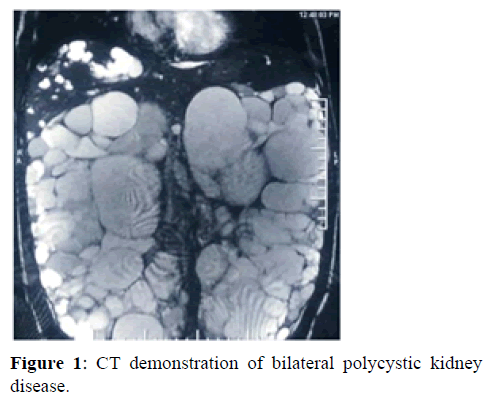
Role of polycystins, PKD proteins, genes and cellular signaling: Polycystins have a dominant role in cell adhesion and extracellular matrix regulation. The interaction between polycystins is important. They have effects on clonal cellular proliferation, increased apoptosis, abnormal epithelial cell phenotype, extracellular matrix proliferation and inflammation. PKD proteins control cell homeostasis and converging signaling pathways. Control alterations in normal functioning of PKD proteins causes damaging effects on normal cell homeostasis and converging signalling pathways, such as Ca2+, cAMP, WNT, vascular endothelial growth factor and Hippo signalling [3,4]. The role of PKD proteins on multiple aspects of development especially during embryonic life on renal tissue maturation has pleotrophic effects. The role of Ca2+, cAMP, WNT, vascular endothelial growth factor and hippo signaling is critical in normal kidney development. Abnormalities here result in renal dysplasia leading to cyst formation. Wnt signalling is important for normal nephron development [5,6]. PKD1, PKD2 and hepatocyte nuclear factor 1β are considered significant genes in development of kidney normally. PKD1 and PKD2 are expressed in many tissues [7]. Genetic Mutations of PKD1 (chromo-some 16p13.3) are responsible for almost 80% of cases of ADPKD, whereas 15% of ADPKD cases are attributed to mutations in PKD2 (chromosome 4q22.1) and the remaining 5-10% of ADPKD cases are genetically unresolved or are due to rare mutations in other loci. Hepatocyte nuclear factor appears on day 10 of embryonic life [8,9]. Though the name hepatocyte nuclear factor 1β, it has an important role in development of renal system and defects in it causes cystogenesisr. It is also responsible for development of diabetes syndrome in addition to renal cyst formation [10]. It is a transcription factor that upregulates the expression of multiple PKD associated genes, including PKD1 and PKD2, neutral beta-glucosidase AB (GANAB which is involved in protein folding) [11] and DNAJB (which encodes a chaperone protein that is associated with binding-immunoglobulin protein (BIP also known as HSPA5). In addition, ADPKD can result from mutations in genes that are primarily associated with Autosomal Dominant Polycystic Liver Disease (ADPLD), including SEC63 (which encodes a protein that is required for protein translocation across the Endoplasmic Reticulum (ER) membrane). Alterations in functioning of neutral beta-glucosidase AB, DNAJB11 SEC63 cause defects at various levels preventing normal cellular cohesion, adhesion, interaction, promotion of dysplasia causing disorganized cellular proliferation leading to development of cysts within renal tubular cells. Patients with mutations in GANAB can manifest with an ADPKD or ADPLD phenotype, and other possible ADPLD causing genes include LRP5 (which encodes a WNT co-receptor) ALG8 and SEC61B). LRP5, ALG8 and SEC61B dysfunction is related to cyst formation. Interaction between cilia and polycystins is critical for cyst development. Abnormal interaction leads to Cyst development. Fibroblast Growth Factors (FGF) also regulate growth. It has been seen that FGF 23 levels are higher in patients of ADPKD patients [12,13]. Transforming Growth Factors (TGF) also regulate growth. It plays a dominant role in signal transduction [14]. It has been seen that TGF beta levels are higher in patients of ADPKD patients. It is not only the kidneys which may be affected by such unorganized and clonal cellular proliferation but other organs as well. In polycystic kidney disease, cysts may be present in other organs as well such as liver, spleen and pancreas. The carriers with a mutation in one PKHD1 allele may also sometimes manifest with a few liver or kidney cysts. Though cysts at other places are not that frequent. Dysregulated ciliary dependent signalling can be caused by mutations in Uromodulin (UMOD), mucin 1 (MUC1) Renin (REN). Mutations in COL4A1 (which encodes a collagen subunit), including those associated with Hereditary Antipathy with Nephropathy, Aneurysms And Muscle cramps (HANAC) syndrome can sometimes cause an ADPKD like phenotype (Figure 1).
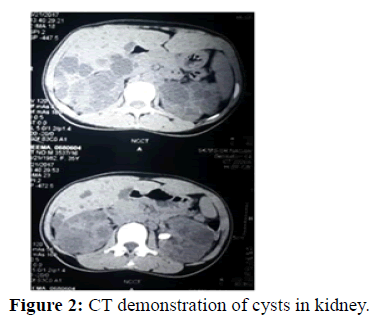
Role of cilia: Defects in cilia along with genetic mutations are believed to be causative in the two main forms of monogenic cystic kidney diseases. Cilia line the nephron and pancreatic ducts [15]. The human cilia are a diverse structures with multiple functions. In kidneys they have a mechanosensory function [16]. Cilia in embryonic life are concerned with normal organogenesis and organ rotation. These cilia play critical roles in tissue development and signal transduction. Mutations or defects in ciliary associated proteins result in a ciliopathies. The cilia protrude in to the lumen of renal tubular epithelial cells especially of proximal tubules, distal tubules, and loop of Henle (Figure 2).
Discussion
These cilia act as mechanosensors as well as chemosensory for determining fluid composition, movement along with calcium uptake. Ciliary abnormalities in embryonic life disrupt fluid uptake leading to fluid accumulation and cyst formation. The normal primary cilium is a membrane covered tubular structure with a highly organized array of microtubules. The ultrastructure of the mammalian cilium is 9+2. Polycystin-1, polycystin-2, and fibrocystins are mainly seen to be localized to the primary cilium in kidney especially in the renal epithelial cells. They mediate via cAMP pathway [17]. Polycystin 2 is a calcium permeable ion channel [18]. The polycystins, and fibrocystin, are involved in the ciliary mediated response to flow by initiating intracellular calcium release. This flow response is a result of cilium activation, apparently from a low fluid shear that causes the cilium to bend, with subsequent activation of the polycystins and other interacting proteins in this complex along with the downstream signaling components. It has been seen that the polycystin-1 binds to various heterotrimeric Gproteins and the signal transduction arising from their activation may contribute to the overall cellular response to flow. The non-calcium mediated signaling pathways may possibly bridge ciliary information to the polycystins at other subcellular localizations. Renal ciliopathies lead to development of cysts. Ciliopathies result in cystic kidneys and are characterized by the presence of kidney cysts that develop due to uncontrolled epithelial cell proliferation, growth, and polarity, downstream of dysregulated ciliary dependent signaling. Uncontrolled epithelial cell proliferation is fundamental to cyst formation. The degree of proliferation would determine the size of cysts and the number of cysts. Even the location and distribution of cysts would be genetically controlled in most cases. Dysregulated ciliary dependent signalling is considered a fundamental process in embryonic period causing cyst formation. Failure of embryonic kidney elements to fuse as a result of inappropriate signalling leads to cyst formation. Other associations of polycystic kidney disease are intracranial aneurysms, hepatic and pancreatic cysts, arachnoid cysts, diverticular disease, abdominal hernias and bronchiectasis [19-21].
Conclusion
Knowing the embryological basis, understanding the defects in cilia, polycystins and cellular signaling, future genetic diagnosis might benefit patients, families and improve the clinical management of patients with polycystic kidney disease reducing progression to kidney failure requiring dialysis and transplantation. It is important to determine the role of cilia, polycystins, cellular signalling defects, genetic mutations in cyst initiation, formation and to devise effective modalities for preventing the development of cystic disease of kidneys.
References
- Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest. 1991;64:777-784. [Google Scholar] [PubMed]
- Atala A. Congenital anomalies of the kidney. In: Schrier RW (ed). Diseases of the Kidney and Urinary Tract, Philadelphia, 7th ed, Lippincott William and Wilkins, USA, 2001;649-662.
- Wilkes M, Madej MG, Kreuter L. Molecular insights into lipid-assisted Ca2+ regulation of the TRP channel polycystin-2. Nat Struct Mol Biol. 2017;24:123-130. [Crossref] [Google Scholar] [PubMed]
- Liu X, Vien T, Duan J. Polycystin-2 is an essential ion channel subunit in the primary cilium of the renal collecting duct epithelium. Life. 2018;75. [Crossref] [Google Scholar] [PubMed]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283-292. [Crossref] [Google Scholar] [PubMed]
- Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, et al. Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol. 2007;293:494-500. [Crossref] [Google Scholar] [PubMed]
- Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339-1342. [Crossref] [Google Scholar] [PubMed]
- Ott MO, Rey Campos J, Cereghini S, Yaniv M. vHNF1 is expressed in epithelial cells of distinct embryonic origin during development and precedes HNF1 expression. Mech Dev 1991;36:47-58. [Crossref] [Google Scholar] [PubMed]
- Barbacci E, Reber M, Ott MO, Breillat C, Huetz F, Cereghini S. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development. 1999;126:4795-805. [Crossref] [Google Scholar] [PubMed]
- Montoli A, Colussi G, Massa O, Caccia R, Rizzoni G, Civati G, et al. Renal cysts and diabetes syndrome linked to mutations of the hepatocyte nuclear factor-1 beta gene: description of a new family with associated liver involvement. Am J Kidney Dis. 2002;40:339-402. [Crossref] [Google Scholar] [PubMed]
- Porath B, Gainullin VG, Gall EC. Mutations in GANAB, Encoding the Glucosidase IIa Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. Am J Hum Genet. 2016;98:1193-1207. [Crossref] [Google Scholar] [PubMed]
- Wanders E, Morsche RHT, Man RAD, Jansen JBMJ, Drenth JPH. Extensive mutational analysis of PRKCSH and SEC63 broadens the spectrum of polycystic liver disease. Hum Mutat. 2006;27:830-838. [Crossref] [Google Scholar] [PubMed]
- Chonchol M, Gitomer B, Isakova T, Cai X, Salusky I, Pereira R, et al. Fibroblast Growth Factor 23 and Kidney Disease Progression in Autosomal Dominant Polycystic Kidney Disease. Clin J Am Soc Nephrol. 2017;12:1461-1469. [Crossref] [Google Scholar] [PubMed]
- Dennler S, Goumans MJ, Dijke PT. Transforming growth factor beta signal transduction, J Leukoc Biol. 2002;71:731-740. [Crossref] [Google Scholar] [PubMed]
- Wheatley D, Wang A, Strugnell G. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73-81. [Crossref] [Google Scholar] [PubMed]
- Schwartz E, Leonard M, Bizios R, Bowser S. Analysis and modeling of the primary cilium bending response to fluid shear. Am J Physiol. 1997;272:132-138. [Crossref] [Google Scholar] [PubMed]
- Belibi FA, Reif G, Wallace DP, Yamaguchi T, Olsen L, Li H, et al. Cyclic AMP promotes growth and secretion in human polycystic kidney epithelial cells. Kidney Int. 2004;66:964-973. [Crossref] [Google Scholar] [PubMed]
- Tsiokas L, Kim S, Ong EC. Cell biology of polycystin-2. Cell Signal. 2007;19:444-453. [Crossref] [Google Scholar] [PubMed]
- Pirson Y, Chauveau D, Torres V. Management of cerebral aneurysms in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2002;13:269-276. [Crossref] [Google Scholar] [PubMed]
- Belz MM, Hughes RL, Kaehny WD. Familial clustering of ruptured intracranial aneurysms in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2001; 38:770-776. [Crossref] [Google Scholar] [PubMed]
- Torra R, Nicolau C, Badenas C. Abdominal aortic aneurysms and autosomal dominant polycystic kidney disease. J Am Soc Nephrol 1996;7:2483-2486. [Crossref] [Google Scholar] [PubMed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.