A Study of Insulin Resistance and its Clinico‑Metabolic Associations Among Apparently Healthy Individuals Attending a Tertiary Care Hospital
- *Corresponding Author:
- Dr. Sharmistha Bhattacherjee
Department of Community Medicine, North Bengal Medical College and Hospital, Siliguri - 734 012, West Bengal, India.
E-mail: sharmistha.bhattacherjee@ gmail.com
Abstract
Background: Insulin resistance (IR), as a result of unhealthy life‑styles and westernization, most likely contributes to the increased incidence of metabolic abnormalities and consequently, the development of metabolic syndrome (MS). Aim: The present study was undertaken to determine the magnitude of IR and associated clinico‑metabolic risk factors among the out‑patients of a tertiary care hospital in Bihar, India. Subjects and Methods: Anthropometric profile, lipid profile, fasting blood glucose, C‑reactive protein (CRP) and C‑peptide of 112 individuals were measured using the standard procedures. IR was assessed using the homeostasis model (Homeostatic model assessment [HOMA]‑IR). Results: The mean IR was 1.5 (1.0). Individuals with MS, higher body mass index and CRP ≥6 mg/l had higher IR. Linear regression showed, among the components of MS, waist circumference had the highest contribution toward IR. The optimal cut‑off value to detect IR by HOMA2‑IR was 1.35. Conclusion: IR was found to have a strong association with various clinico‑metabolic risk factors.
Keywords
C-reactive protein, Homeostatic model assessment insulin resistance, Insulin resistance, Metabolic syndrome
Introduction
The new millennium has witnessed the emergence of the epidemic of non-communicable diseases, with frightful consequences to the health of people world-wide. Insulin resistance (IR), defined as a reduced biological action of insulin, has emerged as a major pathophysiological factor in the development and progression of a number of common non-communicable diseases in man including type 2 diabetes mellitus, polycystic ovary disease, dyslipidemia, hypertension, cardiovascular disease, sleep apnea, certain hormone-sensitive cancers and obesity.[1,2]
The concept of IR was proposed as early as 1936[3] and is generally defined as decreased sensitivity or responsiveness to the metabolic actions of insulin, such as insulin-mediated glucose disposal and inhibition of hepatic glucose production.[4]
IR is probably the unifying pathophysiological denominator of a cluster of non-communicable disease risk factors including elevated plasma glucose, lipid regulation problems (elevated triglycerides increased small low-density lipoproteins and decreased high-density lipoproteins), hypertension and obesity. This combination is referred to as either “the metabolic syndrome (MS)” or “syndrome X” or “IR syndrome”.[1]
A lack of accepted criteria and in cognizance of the difficulty in using a surrogate marker (hyperinsulinemia) for IR in clinical practice, the World Health Organization in 1998, more recently NCEP-ATP III [The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III)], proposed the use of a group of lipid and non-lipid risk factors of metabolic origin, which are closely linked to IR.[5,6]
In the Indian context, the increasing tendency of its inhabitants to develop MS due to genetic predispositions and change of life-style due to the impact of westernization and rapid urbanization, has led the people to become more vulnerable to developing IR.[7]
The gold standard test for evaluating IR is the euglycemic hyperinsulinemic clamp in which after overnight fast, insulin is infused intravenously at a constant rate and glucose is infused intravenously at a variable rate to maintain constant glucose concentrations. Its use is limited to clinical practice owing to the time and cost involved.[8] For this reason, simpler, less invasive techniques of determining IR like Homeostatic model assessment (HOMA) have been developed. The technique is a method for assessing β-cell function and IR from basal glucose and insulin or C-peptide concentrations.[9] The original model HOMA1-IR, first demonstrated by Matthews et al. (1985),[10] has been widely used, especially in different epidemiological and clinical studies. However, the model was updated with some physiological adjustments to a computer version (HOMA2-IR) providing a more accurate index.[11]
Though, available literature amply illustrates the correlation of IR with metabolic risk factors limited data is available of its prevalence in the state of Bihar. Keeping this in mind, the present study was undertaken to find out the magnitude of IR and its associated metabolic risk factors among the patients attending the outpatient clinic of the Mata Gujri Memorial Medical College and LSK Hospital, Kishanganj, Bihar.
Subjects and Methods
A hospital-based cross-sectional study was conducted among purposively selected 112 apparently healthy individuals aged 20 years and above, attending the out-patient clinic of the Mata Gujri Memorial and LSK Hospital, Kishanganj, during the period of June 2010 to November 2010. Participants were informed about the purpose of the study and those who gave their consent were requested to visit the hospital on pre-specified dates for blood testing after an overnight fast for 12 h. Patients with previously diagnosed cardiovascular diseases, diabetes mellitus, renal failure, hyperuricemia were excluded from the study. All participants were interviewed by using a pre-tested, pre-designed standard questionnaire that evaluated various socio-demographic, clinical and biological characteristics including age, gender, weight, height, waist circumference and blood pressure (BP). Ethical clearance for this study was obtained from the MGM Medical college Kishanganj Bihar.
Biophysical parameters
Body weight was measured by portable weighing machine setting the pointer at zero reset with the subject wearing light clothes and without shoes. Height was recorded during inspiration using a standardized self-rolling steel plate to the nearest 0.5 cm. Body mass index (BMI) was calculated by dividing the weight of an individual in kilogram by the square of his/her height measured in meters; and was categorized as per as per Indian Council of Medical Research classification (normal: 18.5-22.9 kg/m2, overweight: 23-24.99 kg/m2, obesity: ≥25 kg/m2).[12] BP was recorded using the standard mercury sphygmomanometer with adult size cuff in palpatory method first then the auscultatory method in a sitting posture. Three measurements were taken at the interval of 5 min; the mean value was taken among the three systolic BP and diastolic BP respectively. Waist circumference was assessed at the end of expiration, measuring the minimum circumference at the level of the umbilicus to the nearest 0.1 cm. For measurement in female subjects, a female attendant was taken to stand by the side.
Biochemical measurements
The blood samples were collected from the antecubital vein after 12 h of fasting and avoiding of alcohol. The level of serum glucose, high density lipoprotein cholesterol (HDL-C), triglyceride, were measured by Erba CHEM-5 Plus V2 auto-analyzer [Transasia Bio-Medicals Ltd]. The plasma sugar was determined using the glucose oxidase enzymatic method (Trinder, 1969). Triglyceride concentration was determined with a semi-automated enzymatic analyzer (RA 50, Semi-auto Chemistry Analyzer, Thyrocare India Ltd., India). Serum HDL-C level was measured by using the phosphotungstate precipitation method. Plasma C-reactive protein (CRP) was estimated using the latex slide test based on an immunologic reaction between CRP as an antigen and latex particles coated with non-specific anti human CRP and is sensitized to detect levels greater than 6 μg/ml CRP. C-peptide was estimated by Acculite™ chemiluminescence immuno assay (CLIA) test system based on CLIA method. An internal quality control was in place for assessing the validity of glucose, triglyceride and HDL methods.
MS and IR
The International Diabetes Federation criteria[13] was used to define MS. Insulin sensitivity was assessed by the calculation of the HOMA approach. The HOMA2-IR index was obtained by the program HOMA-Calculator version 2.22 [http://www. dtu.ox.ac.uk/] taking into consideration both fasting C-peptide and fasting plasma glucose level.[11]
Statistical analyses
Continuous variables are presented as mean values (standard deviation), while qualitative variables are presented as relative frequencies. Comparisons between normally distributed continuous variables and categorical were performed by the calculation of Student’s t-test and one-way or multi-way analysis of variance, after testing for equality of variances (homoscedasticity) using IBMSPSS [Statistical Package for the Social Sciences] version 20 software (Chicago, IL, USA). Linear regression analysis was carried out using constant, waist circumference, serum HDL-C, systolic BP in mm of Hg, fasting blood glucose, serum triglycerides, diastolic BP in mm of Hg as predictor variables and HOMA2-IR as the dependent variable. All reported P values are based on two-sided tests and compared with a significance level of 5%. SPSS version 16.0 was used for all statistical analyses.
The cut-off values for IR were based on the 90th percentile in the study population and a receiver operating characteristic (ROC) curve was generated. The area under the ROC curve was calculated to evaluate the accuracy of the HOMA2-IR index. The optimal cut-off value was denoted by the value that had the largest sum of sensitivity and specificity.
Informed consent was obtained from the participants. Participation in the study was voluntary and guarantee of confidentiality and anonymity of data was ensured. Ethical clearance was obtained from the Institutional Ethics Committee.
Results
Background factors of subjects categorized by sex
Among the 112 individuals who participated in the study, there were 43 males and 69 females. The mean and standard deviation of both biological and biochemical parameters between males and females were calculated and presented in Table 1. Males had a higher waist circumference, diastolic BP while females had a higher BMI, systolic BP, serum HDL, CRP and C-peptide levels. The parameters for assessment of IR by HOMA2-IR among the study population were studied and it was found that the fasting plasma glucose had a mean 91.7 (18.9) and C-peptide had mean 2.1 (1.8). HOMA2-IR calculated from these indices shows mean 1.5 (1.0).
| Parameters | Male (n=43) | Female (n=69) | Total | P value* | |||
|---|---|---|---|---|---|---|---|
| Age | 42.6 | (13.92) | 42.8 | (14.41) | 42.7 | (14.12) | P=0.95 |
| BMI (kg/m2) | 21.9 (3.81) | 24.4 (7.21) | 23.4 (6.23) | P=0.04 | |||
| Waist circumference (cm) | 83.3 | (11.01) | 79.0 | (12.61) | 80.7 | (12.24) | P=0.06 |
| Fasting blood glucose (mg/dl) | 91.9 | (23.53) | 91.5 | (15.62) | 91.7 | (18.91) | P=0.92 |
| Systolic blood pressure (mm of Hg) | 122.9 (27.21) | 126.1 (21.83) | 124.9 (24.01) | P=0.49 | |||
| Diastolic blood pressure (mm of Hg) | 80.1 (8.43) | 77.4 | (13.14) | 78.5 | (11.61) | P=0.19 | |
| Triglycerides (mg/dl) | 106.2 (51.14) | 106.6 (42.81) | 106.4 (45.90) | P=0.96 | |||
| HDL (mg/dl) | 33.0 (6.24) | 39.2 (6.61) | 36.8 (7.11) | P<0.001 | |||
| Serum C-peptide | 2.0 | (1.92) | 2.1 | (0.82) | 2.1 | (1.32) | P=0.55 |
| CRP | 2.5 | (2.61) | 3.6 | (8.01) | 3.2 | (6.52) | P<0.01 |
| Insulin resistance by HOMA2-IR | 1.5 | (1.11) | 1.7 | (0.82) | 1.5 | (1.03) | P=0.22 |
Data presented mean±SD. *P value from Student’s t-test for continuous normally distributed variables; Mann-Whitney U-test for skewed distributions. HOMA: Homeostatic model assessment, SD: Standard deviation, BMI: Body mass index, HDL: High density lipoprotein, CRP: C-reactive protein
Table 1: Biological and biochemical parameters of the study population (n=112)
IR of subjects categorized by BMI, CRP and MS
It is evident from Table 2 that the mean IR using HOMA2-IR in individuals with normal BMI was 1.1 (0.4) and 2.2 (0.8) among the obese; 2.0 (0.9) among those with CRP ≥6 mg/l and 2.3 (1.0)± in persons with MS. Distribution of IR using HOMA2-IR varies significantly with BMI, CRP and occurrence of MS and there is no statistically significant difference in mean and standard deviation of IR (HOMA2-IR) among males (1.5 [1.1)]) and females (1.7 [0.8]).
| Variables | n | HOMA2-IR | Test | |
|---|---|---|---|---|
| Mean | SD | |||
| BMI | ||||
| Normal | 52 | 1.07 | 0.43 | F=24.753, P<0.001 |
| Overweight | 22 | 1.97 | 1.25 | |
| Obese | 38 | 2.18 | 0.82 | |
| CRP | ||||
| <6 mg/l | 87 | 1.52 | 0.91 | t=−2.228, P=0.03 |
| ≥6 mg/l | 25 | 1.99 | 0.95 | |
| MS | ||||
| Yes | 41 | 2.36 | 1.05 | t=6.646, P<0.001 |
| No | 71 | 1.20 | 0.51 | |
HOMA: Homeostatic model assessment, IR: Insulin resistance, SD: Standard deviation, BMI: Body mass index, CRP: C-reactive protein, MS: Metabolic syndrome
Table 2: Distribution of insulin resistance using HOMA2-IR among the study population (n=112)
Relationship between components of MS and IR
From the regression results, it was observed that among the components of MS, waist circumference had the highest contribution toward the dependent variable IR, followed by serum triglycerides, fasting blood glucose, serum HDL-C, systolic BP in mm of Hg and lastly, diastolic BP in mm of Hg. The regression model for this purpose can be best expressed as follows:
IR = −2.169 + 0.26 (waist circumference) +0.008 (serum triglycerides) +0.004 (serum HDL-C) +0.005 (fasting blood glucose) +0.003 (systolic BP in mm of Hg) −0.001 (diastolic BP in mm of Hg).
Applying the above model, 38.9% of the total variation of IR can be predicted using the variables waist circumference, serum HDL-C, systolic BP in mm of Hg, fasting blood glucose, serum triglycerides, diastolic BP in mm of Hg [Table 3].
| Statistics | Variables | Regression coefficient | Significant | 95% confidence Interval | |
|---|---|---|---|---|---|
| R=0.650 | (Constant) | −2.169 | |||
| Adjusted | Serum triglycerides | 0.01 | <0.001 | 0.005 | 0.012 |
| R2=0.389 | Serum HDL cholesterol | 0.004 | 0.67 | −0.016 | 0.024 |
| Fasting blood glucose | 0.005 | 0.22 | −0.003 | 0.013 | |
| Systolic BP in mm of Hg | 0.003 | 0.49 | −0.005 | 0.011 | |
| Diastolic BP in mm of Hg | −0.001 | 0.87 | −0.018 | 0.015 | |
| Waist circumference | 0.03 | <0.01 | 0.012 | 0.039 | |
Predictors: (Constant), waist circumference, serum HDL cholesterol, systolic BP in mm of Hg, fasting blood glucose, serum triglycerides, diastolic BP in mm of Hg. Dependent variable: HOMA2-IR. HDL: High density lipoprotein, MS: Metabolic syndrome, BP: Blood pressure, HOMA: Homeostatic model assessment, IR: Insulin resistance
Table 3: Linear regression for predicting insulin resistance by the components of MS (n=112)
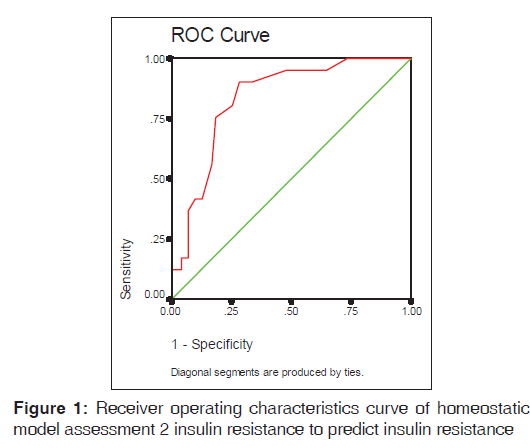
Areas under ROC curves (95% confidence interval [CI]) to predict IR using HOMA2-IR
Figure 1 shows that HOMA2-IR index presented area under the curve of 0.834 with overlapping 95% CI of 0.758-0.909. Using the ROC curve analysis the optimal value for sensitivity and specificity that keep (1 − sensitivity) + (1 − specificity) at minimum was 1.35. Sensitivity and specificity were 90.2% and 71.8%, respectively.
Discussion
IR has been suggested as the primary cause leading to the clustering of risk factors such as glucose intolerance, hypertension, elevated serum triglycerides, low serum HDL-C and central obesity which together have been labeled as MS.[14] Direct measurement of IR using the hyperinsulinemic-euglycemic clamp is the gold standard, but it has limited practical application. Previous studies have shown that HOMA-IR based IR measurements have a strong correlation with glucose clamp-assessed IR.[10,15]
In the present study, IR was calculated by HOMA2-IR method, which is a more accurate representation of metabolic process because it models the feedback relationship between insulin and glucose in various organs of the body.[9]
The present study demonstrated the magnitude of IR and its associated metabolic risk factors among the 112 attendees of an out-patient department of a tertiary care hospital. There were 43 males and 69 females and the mean age of the study population were 45.9 (13.6) years.
| Area under curve | Significant | 95% confidence interval | Cut-off value at 90th percentile | Sensitivity | Specificity |
|---|---|---|---|---|---|
| 0.834 | <0.001 | 0.758-0.909 | 1.35 | 90.2% | 71.8% |
Table: Insulin resistance by HOMA2-IR
The differences between males and females were evident in the study population despite similarities in mean age, fasting blood glucose, triglycerides and C-peptide. The mean IR as calculated by HOMA2-IR was 1.5 (1.0) which was very close to the findings of the study among Pakistani subjects using HOMA-IR method by Hydrie et al. (1.6 [0.8]).[16]
In a study, among 1525 Peruvian adults by Gelaye et al., it was found that the mean value of waist circumference, systolic and diastolic BP, triglycerides were significantly higher among males. Women had significantly higher mean age and high density lipoprotein-cholesterol; there was no significant gender difference in the values of CRP, fasting insulin and fasting glucose.[17]
In the present study, the gradient of IR has increased significantly with increasing categories of BMI and CRP. CRP, a systemic inflammatory marker, when measured in the blood with high sensitivity assay has been reported to be a strong and independent predictor of future cardiovascular disease (CVD) risk including IR.[18,19]
Gelaye et al.[17] in their study demonstrated elevated CRP levels significantly associated with increased mean fasting insulin and mean HOMA-IR concentrations, whereas, in a study done by Moran et al. among the youths of Minneapolis, CRP levels in children age 10-16 years were found not to be significantly associated with IR.[20] On the other hand, Vikram et al. in a study among the urban young adult north Indian males, failed to show any correlation between highly sensitive CRP and IR.[21]
The relationship of obesity to IR and type 2 diabetes is a long-recognized phenomenon with fundamentally important scientific and clinical implications.[22] Changes in BMI were found to be positively correlated with changes in IR in a study among black and white adolescent girls by Auwerx et al. (P < 0.001)[23] and in healthy Brazilian subjects by Geloneze et al.[24] which was in consonance with the present study. In contrast, in a study among Sri Lankan diabetic population, a negative correlation was found between BMI and IR by HOMA-IR.[25]
According to Reaven, IR is the central pathophysiological feature of the cluster of metabolic abnormalities, which are associated with MS.[14] Globally, several studies have suggested that subjects with MS are more insulin resistant and are at increased risk for CVD than those without MS[26,27] which are similar to the findings of the present study.
Finally, to confirm whether the risk factors could be attributed to enhanced IR, HOMA-IR in comparison with those of MS components (waist circumference, fasting blood glucose, systolic BP/diastolic BP, serum triglycerides and HDL-C) were examined and it was observed that serum triglycerides and waist circumference were significantly related to IR. Similar findings were observed by Yamada et al.[28] and Kawamoto et al.[29] among the Japanese population.
The presence of hypertriglyceridemia always associated with IR in these studies may be due to the fact that insulin affects triglyceride and HDL-C metabolism.[30].
Determining cut-off values of IR by indirect measures could help in identifying insulin resistant subjects in clinical practice on account of their simplicity and clinicians may be able to use this simple test as an initial screening tool to identify such subjects in the future. The ROC curve [Figure 1] in the present study depicts that the optimal cut-off value to detect IR by HOMA2-IR was 1.35. The optimal cut-off value to detect IR was 1.8 among Brazilian population[24] and 1.67 among the Argentinean population.[26]
The strength of the study is that it is one of the first study in this part of the country which measures IR based on HOMA2-IR among apparently healthy individuals. However, the limitations of our study must also be considered. The number of subjects studied was very small and they may not be representative of the general population. Due to the cross-sectional nature of the present study, the cause-effect relationship of our findings cannot be proven and a large scale, prospective study is required.
Conclusion
The findings of the present study reveal that IR is associated with various clinico-metabolic risk factors. In conclusion, IR can be viewed as a large iceberg where unknown morbidity exceeds the known morbidity. The physician recognizes only the clinically evident manifestations which reflect only the proverbial “tip of the iceberg” - diabetes, obesity, hypertriglyceridemia, hypertension, diminished HDL cholesterol and atherosclerosis – and the complete IR syndrome may be missed. With the recognition that IR is a multifaceted syndrome that can express itself in many ways, it is important for the scientific community to focus their attention on defining the mechanism (s) responsible for this defect.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Kelly GS. Insulin resistance: Lifestyle and nutritional interventions. Altern Med Rev 2000;5:109-32.
- Krentz AJ. Insulin resistance in clinical medicine. In: Insulin Resistance: A Clinical Handbook. Oxford, UK: Blackwell Science Ltd.; 2008.
- Himsworth HP. Diabetes mellitus: Its differentiation into insulin-sensitive and insulin-insensitive types. Lancet 1936;227:127-30.
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15-26.
- LeRoith D. Beta-cell dysfunction and insulin resistance in type 2 diabetes: Role of metabolic and genetic abnormalities. Am J Med 2002;113 Suppl 6A: 3S-11.
- National Center for Health Statistics. Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988-1994. Department of Health and Human Services Publication No. (PHS) 94-1308 (Vital and Health Statistics; Series 1, No. 32). Hyattsville, MD: National Center for Health Statistics; 1994.
- Misra A, Misra R. Asian Indians and insulin resistance syndrome: Global perspective. Metab Syndr Relat Disord 2003;1:277-83.
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214-23.
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487-95.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412-9.
- Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191-2.
- Mohan V, Deepa R. Obesity and abdominal obesity in Asian Indians. Indian J Med Res 2006;123:593-6.
- International Diabetes Federation (IDF). The IDF consensus worldwide definition of the metabolic syndrome. Available from: http://www.idf.org/webdata/docs/ IDF_Metasyndrome_definition.pdf. [Last accessed on 2013 Apr 06].
- Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595-607.
- Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care 2000;23:171-5.
- Hydrie MZ, Basit A, Fawwad A, Ahmedani MY, Shera AS, Hussain A. Detecting insulin resistance in Pakistani subjects by fasting blood samples. Open Diabetes J 2012;5:20-4.
- Gelaye B, Revilla L, Lopez T, Suarez L, Sanchez SE, Hevner K, et al. Association between insulin resistance and c-reactiveprotein among Peruvian adults. Diabetol Metab Syndr 2010;2:30.
- Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: Moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007;49:2129-38.
- Nakanishi N, Shiraishi T, Wada M. Association between C-reactive protein and insulin resistance in a Japanese population: The Minoh Study. Intern Med 2005;44:542-7.
- Moran A, Steffen LM, Jacobs DR Jr, Steinberger J, Pankow JS, Hong CP, et al. Relation of C-reactive protein to insulin resistance and cardiovascular risk factors in youth. Diabetes Care 2005;28:1763-8.
- Vikram NK, Misra A, Pandey RM, Dwivedi M, Luthra K, Dhingra V, et al. Association between subclinical inflammation and fasting insulin in urban young adult north Indian males. Indian J Med Res 2006;124:677-82.
- Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473-81.
- Auwerx J, Schoonjans K, Fruchart JC, Staels B. Regulation of triglyceride metabolism by PPARs: Fibrates and thiazolidinediones have distinct effects. J Atheroscler Thromb 1996;3:81-9.
- Geloneze B, Vasques AC, Stabe CF, Pareja JC, Rosado LE, Queiroz EC, et al. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq Bras Endocrinol Metabol 2009;53:281-7.
- Hettihewa LM, Dharmasiri LP, Ariyaratne CD, Jayasinghe SS, Weerarathna TP, Kotapola IG. Significant correlation between BMI/BW with insulin resistance by McAuley, HOMA and QUICKI indices after three months of pioglitazone in diabetic population. Int J Diabetes Dev Ctries 2007;27:87-92.
- Buccini GS, Wolfthal DL. Cutoff values for indices of insulin resistance, insulin sensitivity and insulin secretion derived from the formula HOMA and HOMA2 program. Interpretation of the data. Rev Argent Endocrinol Metab 2008;45:3-21.[Article in Spanish]
- Hsu CH. Different impacts of metabolic syndrome components on insulin resistance in type 2 diabetes. Int J Endocrinol 2013;2013:740419.
- Yamada C, Moriyama K, Takahashi E. Association between insulin resistance and metabolic syndrome risk factors in Japanese. J Diabetes Investig 2012;3:185-90.
- Kawamoto R, Tabara Y, Kohara K, Miki T, Kusunoki T, Takayama S, et al. Relationships between lipid profiles and metabolic syndrome, insulin resistance and serum high molecular adiponectin in Japanese community-dwelling adults. Lipids Health Dis 2011;10:79.
- Lewis GF, Uffelman KD, Szeto LW, Steiner G. Effects of acute hyperinsulinemia on VLDL triglyceride and VLDL apoB production in normal weight and obese individuals. Diabetes 1993;42:833-42.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.