A Study on Oral Mucosal Lesions in 3500 Patients with Dermatological Diseases in South India
- *Corresponding Author:
- Dr. Arvind Babu RS
Lecturer in Dentistry, Faculty of Medical Sciences – Dental Programme, The University of West Indies, Mona, Kingston – 7, Jamaica, West Indies
E-mail: arvindbabu2001@gmail.com
Abstract
Background: Oral mucosal lesions that are observed in the dermatological diseases are categorized under mucocutaneous conditions. The oral lesions in dermatological diseases may be the early aspects of the disease manifestation or the most significant clinical appearance or the only sign/and or symptom of such dermatological diseases and occasionally lesions occur simultaneously in the skin as well as mucous membrane. Aim: This present study attempts to find out the prevalence of oral mucosal lesions in patients with dermatological diseases. Subjects and Methods: The study includes 3500 patients who attended out‑patient Department of Dermatology. Patients with oral manifestation were subjected for clinical examination in the Department of Oral Pathology. Diagnostic procedures were performed to confirm the clinical oral diagnosis. The results of the study were analyzed by SPSS software version 19.0 (Armonk, NY) and presented as descriptive statistics. Correlation of oral manifestions with their respective dermatological disease was statistically analysed by Pearson’s correlation test.(P < 0.05 were considered as statistically significant) Results: The prevalence rate of oral mucosal lesions in the present study was 1.8% (65/3500). The most frequent lesions observed were psoriasis 32.3% (21/65), lichen planus 18.4% (12/65), Stevens Johnson Syndrome 18.4% (12/65), pemphigus 10.7% (7/65), toxic epidermal necrolysis 4.6% (3/65), systemic lupus erythematosus 3% (2/65), discoid lupus erythematosus 1.5% (1/65), pemphigoid 1.5% (1/65). Gender distribution in the study population was statistically significant (P < 0.001). Employed and unemployed individuals in the study population were statistically significant (P < 0.001). Pearson’s correlation analysis of oral manifestations with their respective dermatological disease showed r = 0.466 and signifies a positive correlation and is statistically significant at the 0.01 level (two‑tailed). Conclusion: The prevalence rate of oral mucosal lesions in patients with dermatological diseases was relatively low. However, predominant oral mucosal lesions observed in the study were autoimmune in origin with a high morbidity and mortality index. Hence, multidisciplinary approach will definitely help in the prognosis of patients.
Keywords
Autoimmune disease, Dermatological manifestations, Immunofluorescence, India, Oral lesions, Prevalence
Introduction
Traditionally, mucosal membrane of the oral cavity has been looked upon as mirroring the general health. Some systemic diseases presents with local symptoms and/or lesions in the oral mucosa.[1] From a histological and embryological point of view, the mucosa of the oral?cavity is similar to the skin, but it is subjected to a more complex and inconstant environment. This complex nature modifies the pattern of disease presentation in mouth.[2] In the majority of cases, the earliest signs of ill?health are to be read in the skin and accessible mucous membranes. Dental surgeons and Dermatologists possess this advantage in common that they can actually see the parts of the body, which they are called upon to treat.[3]
Mucocutaneous conditions are a group of disorders mainly observed in dermatology practice. Oral mucosal manifestation may be the initial feature, most florid clinical feature or the only sign of such disease and sometimes lesions occur in both skin and mucus membrane.[4] Vesicle and bullae are the most common clinical presentation of these diseases.[5] Predominantly mucocutaneous conditions were related to autoimmune disorders. The concept of autoimmune disease is based on the premise that the patient’s immune response loses the ability to distinguish between “self” and “non – self.” Usually, the oral vesiculobullous diseases represent the organ specific type of autoimmune diseases in which the oral mucosa and skin are the target tissues.[6]
Vesiculo bullous lesions frequently present diagnostic problems, because these lesions often resemble each other clinically as well as during routine histological examination and thus difficult to differentiate them. Immunofluorescence is a histochemical laboratory staining technique used for demonstrating the presence of antibodies bound to antigens in tissues (Direct immunofluorescence) or circulating body fluids (Indirect immunofluorescence). These techniques are essential to supplement clinical findings and histopathology in the diagnosis of immunobullous disorders. They permit early diagnosis, treatment and subsequent monitoring of disease activity in patients with this potentially life?threatening disorders.[7]
Most of the time, oral manifestations may be found as an initial symptom. In this context, dental surgeons have a possibility to observe disease at the preliminary stage. This perception pre?disposes the dentist in the diagnostics and treatment interaction for mucocutaneous diseases with Dermatologist. Considering the special care and treatment needs required to mucocutaneous diseases due to morbidity and mortality factors an early intervention should be planned. This interdisciplinary approach also reinforces the treatment standards as well as better prognosis of the disease may be expected.
In our literature search, we found only three published papers about the prevalence of oral mucosal lesions in dermatological diseases. Ramírez?Amador et al. in 2000, suggested the frequency of oral conditions in dermatology clinic was 2.8%.[4] Goncalves et al. in 2009, evaluated 88 patients with dermatological diseases and suggested the frequency of oral mucosal lesions was 35.7%.[8] Suliman et al. in 2001, observed the prevalence of oral mucosal lesions in patients with dermatological diseases was 57.9% in Sudanese population.[9] Previous literatures documented the frequency of oral condition in dermatology clinic; however, the diagnostic approach is necessary to confirm the diagnosis. The present study attempts to document the prevalence of the oral mucosal lesions in patients with dermatological lesions and diagnosis were confirmed by histopathological examination and Immunofluorescence diagnostic procedures were done in autoimmune conditions. This precludes a remarkable amount of dermatological diseases that can show their manifestations in the oral cavity and sometimes oral manifestations may be pathognomic part of the disease process. Documenting the frequency of oral mucosal lesions in dermatological diseases may alert the dental surgeons and gives scope for early approach for such disease process and a multidisciplinary approach. The present study aims to document the prevalence of oral mucosal lesions in 3500 patients with dermatological diseases in Guntur, South India.
Subjects and Methods
Subject selection
The study group consisted of 3500 patients, who visited the Outpatient Department of Dermatology, Government General Hospital, between January 2012 and July 2012. Patients with oral lesions were subjected for systematic clinical examination in the Department of Oral and Maxillofacial Pathology. After the clinical diagnosis, Cytologic/histopathologic/ immunofluorescence diagnostic procedures were performed as per the requirement.
Institutional Ethical Board, Institutional Review Board (IRB) approval, patients consent as per the IRB guidelines were obtained for each part of this study and even followed the declaration of Helsinki.
Clinical evaluation
All patients with oral lesions were examined by research workers and data were further validated by the consultants. A thorough history was taken, clinical and systemic examination was performed by the researcher and the findings were recorded in Standard format.
Histological examination
For cases with oral lesions, cytological smear were obtained and stained with routine hematoxylin and eosin method. The biopsy was taken under local anesthesia on an out?patient basis by researcher after patient’s willingness. For the cases with autoimmune diseases two biopsy samples were taken, one sample for routine histological examination and the other for immunofluorescent technique. The final definitive diagnosis was based on histopathological examination were given by Oral Pathologists.
The results of the study were analyzed by SPSS software version 19.0 (Armonk, NY) and presented as descriptive statistics. Correlation of oral manifestions with their respective dermatological disease was statistically analysed by Pearson’s correlation test. (P < 0.05 were considered as statistically significant)
Results
The whole study was done in a span of 8 months, during which 3500 patients with dermatological diseases were examined. Of these, 65 patients also presented with oral mucosal lesions.
The total study population falls between the age range of 1?92 years. The maximum number of cases was in the age range of 31?50 years 31.3% (1096/3500) [Table 1]. The distribution of the study population was made in to younger (lesser than or equal to 30 years) and older individuals (greater or equal to 31 years). The age categorization and disease distribution is statistically significant results with (P < 0.001) [Table 1a]. The distribution of the study group on gender showed female predilection 54.3% (1903/3500) [Table 2]. The distribution of the study population according to the gender showed statistically significant results with (P < 0.001). The majority of patients in the study group were laborers 24.7% (867/3500) [Table 3]. Distribution of the study population based on the occupational status employed and unemployed showed statistical significance results with (P < 0.001) [Table 3a]. The children and students population in this study was excluded as they cannot be categorized in the occupational status.
| Age group | No. of persons | Percentage |
|---|---|---|
| 1?13 years | 597 | 17 |
| 14?30 years | 956 | 27.3 |
| 31?50 years | 1096 | 31.3 |
| 51?70 years | 718 | 20.5 |
| 71?90 years | 124 | 3.5 |
| 91?100 years | 9 | 0.2 |
| 3500 |
Table 1: Distribution of study population according to age group
| Distribution of study population according to age(Age in two categories) | Category | N | Observed prop. | Test prop. | Exact significant (two?tailed) |
|---|---|---|---|---|---|
| Younger | ≤30 years | 1553 | 0.44 | 0.50 | 0.001* |
| Older | ≥31 years | 1947 | 0.56 | 0.001* | |
| Total | 3500 | 1.00 |
*P<0.01
Table 1a: Distribution of study population according to age category by Binomial test
| Gender | No. of persons | Percentage |
|---|---|---|
| Male | 1597 | 45.6* |
| Female | 1903 | 54.3* |
| Total | 3500 |
*P<0.01
Table 2: Distribution of study population according to gender
| Occupation | No. of persons | Percentage |
|---|---|---|
| Laborers | 867 | 24.7 |
| House wives | 521 | 14.8 |
| Farmers | 506 | 14.4 |
| Drivers | 493 | 14 |
| Students | 376 | 10.7 |
| Business men | 236 | 6.7 |
| Employed | 226 | 6.4 |
| Children | 147 | 4.2 |
| Unemployed | 128 | 3.6 |
Table 3: Distribution of study population according to occupation
| Occupational status | N | Observed prop. | Test prop. | Exact significant (two?tailed) |
|---|---|---|---|---|
| Employed | 2328 | 0.78 | 0.50 | 0.001* |
| Unemployed | 649 | 0.22 | 0.001* | |
| Total | n=2977 | 1.00 |
*P<0.01
Table 3a: Distribution of study population according to occupational status by Binomial test
Among the 3,500 patients examined, 60 dermatological lesions found in the study. Tinea coporis 17.5% (615/3500) was the most frequent dermatological lesion observed during examination. Oral mucosal lesions were observed in 65 patients. Ten frequently found oral mucosal lesions in the study were listed. Of the 65 patients with oral lesions, psoriasis 32.3% (5 males and 16 females) was the most common. These 10 lesions were 1.8% (65/3500) of all dermatological lesions [Table 4].
| Lesions | No. ofpersons (%) | Male (%) | Female (%) |
|---|---|---|---|
| Psoriasis | 21(32.30) | 5 (23.80) | 16 (76.1) |
| Lichen planus | 12(18.46) | 4 (33.33) | 8 (66.6) |
| Stevens Johnson | 12(18.46) | 6 (50) | 6 (50) |
| syndrome | |||
| Pemphigoid | 8 (12.30) | 0 | 8 (100) |
| Pemphigus | 7 (10.76) | 2 (28.57) | 5 (71.4) |
| Hyperpigmentation | 5(7.69) | 3 (60) | 2 (40) |
| Toxic epidermal | 3(4.61) | 1 (33.33) | 2 (66.6) |
| necrolysis | |||
| Systemic lupus | 2(3.07) | 0 | 2 (100) |
| erythematosus | |||
| Discoid lupus | 1(1.53) | 1 (100) | 0 |
| erythematosus | |||
| Ectodermal dysplasia | 1(1.53) | 0 | 1 (100) |
| 65 | 22 | 43 |
Table 4: Various dermatological lesions with oral mucosal lesions in the study population and gender wise distribution
The percentage of oral mucosal lesions with respective dermatological lesions are toxic epidermal necrolysis 100% (3/3), systemic lupus erythematosus 100% (2/2), discoid lupus erythematosus 100% (1/1), ectodermal dysplasia 100% (1/), pemphigus 100% (7/7), Stevens Johnson Syndrome 85.7% (12/14) psoriasis 17.3% (21/121), lichen planus 16.6% (12/72), pemphigoid 12.5% (1/8) and pigmentation 8.4% (5/59) [Table 5]. Correlation of oral manifestations with their respective dermatological disease showed r value 0.466, which is strong positive correlation under Pearson’s correlation analysis and is statistically significant at the 0.01 level (two?tailed) [Table 5a].
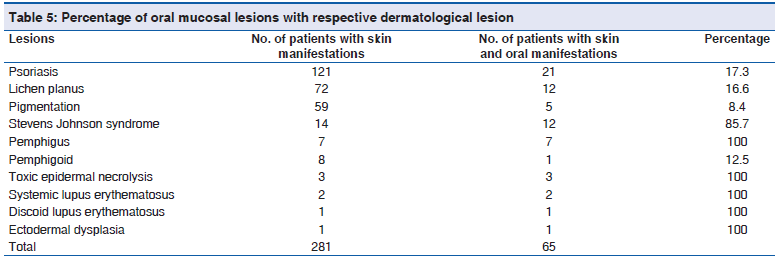
| Distribution of the study population basedon oral mucosal lesions with respectivedermatological lesion | Frequency | Percent | Valid percent | Cumulative percent | Significant (two?tailed) | Pearson’s correlationanalysis (r value) |
|---|---|---|---|---|---|---|
| No. of patients that may have oral | 281 | 8.0 | 8.0 | 100.0 | 0.001 | 0.466* |
| manifestations | ||||||
| No. of patients with skin and oral | 65 | 1.9 | 1.9 | 100.0 | 0.001 | 0.466* |
| manifestations |
*Correlation is significant at the 0.01 level (two?tailed)
Table 5a: Distribution of the study population based on the oral manifestation
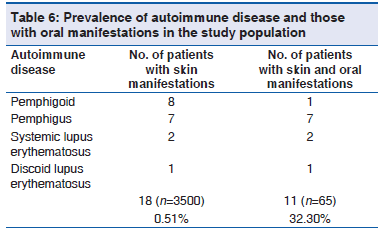
The prevalence of autoimmune disease in the study population was 0.5% (18/3500). The prevalence of oral mucosal lesions showed to be a part of autoimmune disease was 32.3% (11/65). The various autoimmune diseases in the study observed were pemphigoid, pemphigus, systemic lupus erythematosus and discoid lupus erythematosus [Table 6].
Discussion
Prior to discussing the results of the present study, it should be stressed that clinical practice in dermatology may include a great variety of diseases, which affect the oral tissues and those were predominantly autoimmune diseases. Considering the information on autoimmune disease by National Institute of Health reported that autoimmune disorders affect 5?7% of the population with prevalence as high as 20% in the United States.[10] In our study, we observed the prevalence of autoimmune disease was 0.5% of the 3500 patients with dermatological disease. Out of 3500 patients with dermatological disease, 65 patients showed oral mucosal lesions. The prevalence of autoimmune disease in various oral mucosal lesions in dermatological disease was 32.3%. Although a hospital based sample may not represent the actual prevalence of autoimmune disease of the general population, but this study attempts to alert Dermatologist and dental surgeon about the importance of an interdisciplinary approach for diagnosis and management of these life?threatening autoimmune diseases.
It was found that the prevalence of oral mucosal lesions in patients with dermatological disease was 1.82%, with female predilection (54.3%) and mean age range of patients was 1?92 years, with the median age of 38 years. Considering the study by Ramírez?Amador et al. reported that the prevalence of oral conditions in dermatology practice was 2.8% in 2133 patients.[4] Thus, the variation in the prevalence of oral mucosal lesion may be influenced by sample size, geographic distribution, biologic and genetic profile. One possible explanation for female predilection is that, they may be genetically more susceptible for development of diseases.
Our results suggested that 24.7% of patients were laborers. This was consistent with results of Libu et al., who reported that the majority of cases in their study of prevalence and socio?demographic determinants of skin disease were laborers.[11] This could be attributed to hygiene maintenance, socio?economic status of living and may be less health conscious.
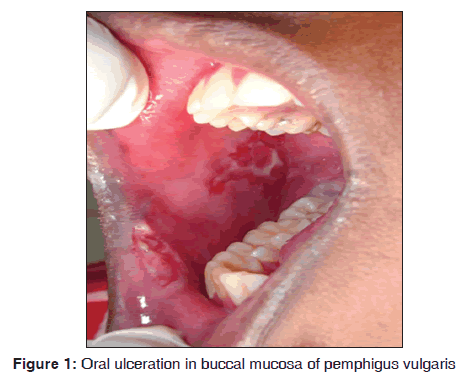
In our study, out of seven pemphigus patients, 5 (71.4%) were females. All the cases showed oral manifestations. The majority of the patients fall in the age group of 30?40 years. Kumar reported incidence of pemphigus was 4.4/million in Kerala population of India and disease exposure was high between 40 and 50 years age group.[12] The oral manifestations initially started as vesicle, which ruptures, leaving a raw eroded ulcerative area. It involves buccal mucosa, tongue and lips [Figure 1]. All cases showed the initial involvement of oral mucosal lesions. Our results suggest that pemphigus was observed in 7/3,500 dermatological patients during 8 months. Shamim et al. stated that oral cavity was the primary site of involvement from his study on clinical analysis of 71 pemphigus patients.[13] Cutaneous lesions showed severe ulcerations. The most common type of pemphigus encountered was pemphigus vulgaris. Out of seven cases, one case of pemphigus vegetans (14.2%) and one case of pemphigus foliaceus (14.2%) were observed. Cytological smear showed the presence of tzanck cells in hematoxylin and eosin staining. Histopathologically, it was characterized by acantholysis, suprabasillar bulla formation. The basal cells lining the floor of the bullae are often arranged in a tombstone pattern and acantholytic keratinocytes float freely within the blister. There were few inflammatory cells in the connective tissue stroma chiefly were lymphocytes. Direct immunofluorescence demonstrated the intercellular fluorescence of immunoglobulin G (IgG) in fish net pattern and complement positivity was seen in all cases. Stanley et al. mentioned that histopathology of pemphigus vulgaris lesions is characterized by acantholysis of epithelial cells with the resultant blister formation just above the basal layer.[14] Anuradha et al. showed deposition of IgG in 86% and C3 positivity in 14% of cases.[15]
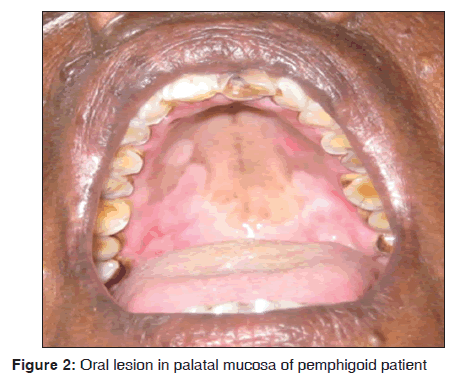
In our study, one out of eight mucous membrane pemphigoid presented with oral manifestations as vesicle and ulcerations over palate [Figure 2], buccal mucosa and gingiva. Skin eruptions showed multiple intact fluid filled blisters of varying size. The age of patient was 60 years. Histopathologically characterized by the presence of sub epithelial bulla and there were few inflammatory cells in connective tissue stroma, chiefly lymphocytes. Direct immunofluorescence demonstrated a linear continuous band of IgG and C3 along the basement membrane zone (BMZ). Our results suggest that bullous pemphigoid was observed in 8/3,500 dermatological patients in 8 months. Adam reported the incidence of bullous pemphigoid in Malaysian population was between 0.2 and 0.3/100,000 persons/year.[16] Gudi et al. reported the incidence of bullous pemphigoid in North East Scotland population was 1.4/100,000 persons/year.[17] Langan et al. reported the incidence of bullous pemphigoid in United Kingdom was 4.28/100,000 persons/year and mortality rate is 19%.[18] Ata?Ali and Ata?Ali suggested that females were commonly involved and belong to age group of fifth?sixth decade of life, with the site predilection of soft and hard palate.[19] Challacombe et al. stated that direct immunofluorescence using perilesional mucosa showed a linear continuous band at the BMZ usually with IgG and C3.[20] Mutasim et al. suggested that in bullous pemphigoid autoantibodies bind to BMZ of stratified squamous epithelium in a linear pattern. This linear pattern can be demonstrated by direct and indirect immunofluorescence. The other patterns are tubular, cytoplasmic and membranous. The pattern of BMZ staining depends on its ultra?structural morphology in each tissue.[21] Anuradha et al. demonstrated a similar pattern of Direct immunofluorescence in 67% of their study subjects.[15]
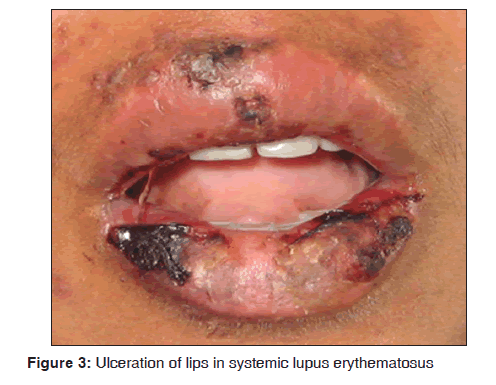
In our study, two female patients of systemic lupus erythematosus were observed. The age range of patients was between 20 and 30 years. They presented with typical Table 4: Various dermatological lesions with oral malar rash, ulcerations over lips [Figure 3], buccal mucosa and palate. Pleural effusion was observed in one of the case and another patient had psychosis. In one case, anti?nuclear antitbodies (ANA) test was positive. Our results suggest that systemic lupus erythematosus was observed in 2/3,500 dermatological patients in 8 months. Malaviya et al. reported the prevalence of systemic lupus erythematosus in Northern Indian population ranges from 14 to 60/100,000 and stated that the prevalence rate of this disease in India is comparatively low.[22] Yacoub Wasef stated that increased prevalence of systemic lupus erythematosus was observed in female patients and the cause was attributed to differences in the metabolism of sex hormones and or gonadotrophin releasing hormone.[23] Jayakumar et al. suggested that oral manifestations showed burning sensation of gingiva, erythematous area over buccal mucosa and white radiating lines near the third molar region. Systemic symptoms such as joint pain and respiratory tract infections were seen.[24] Hochberg stated that cutaneous lesions consists of erythematous patches on the face, which coalesce to form a roughly symmetrical pattern over the cheeks and across the bridge of the nose in a so called butterfly distribution. It also involves neck, upper arms, shoulders and fingers. Revised criteria for classification of systemic lupus erythematosus were malar rash, discoid rash, photosensitivity, oral ulcers, arthritis, serositis, renal disorder, neurologic disorders (seizures, psychosis) and hematologic disorder (hemolytic anemia, leucopenia, lymphopenia and thrombocytopenia).[25] Arbuckle et al. suggested that autoantiboides such as ANA tends to predict the course and progression of systemic lupus erythematosus.[26]
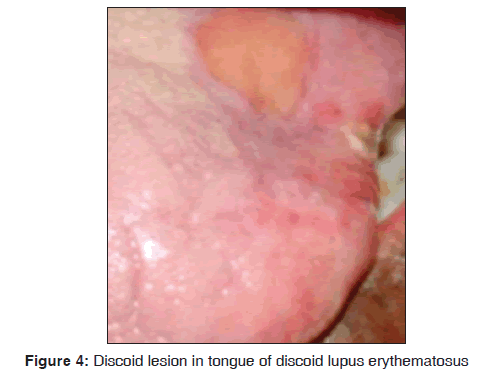
In our study, one 72?year?old male patient with discoid lupus erythematosus was observed. An ulcerative oral lesion was observed over the tongue, lips [Figure 4]. The extra?oral manifestation was butterfly shaped malar rash and discoid rash and no other systemic manifestations were seen. Histopathologically the lesion was characterized by hyperparakeratosis, focal areas of liquefaction degeneration of the basal layer. Direct immunofluorescence demonstrated faint deposition of IgG along the BMZ (Basement Membrane Zone). Our results suggest that discoid lupus erythematosus was observed in 1/3,500 dermatological patients in 8 months. The findings were consistent with previous studies. Pongsiriwet et al. in 2010, stated that discoid lupus erythematosus is a chronic autoimmune disorder of unknown etiology. It can affect the skin and oral mucosa. Classic discoid rashes are frequently found on the sunlight?exposed skin, especially on the face and scalp.[27] Anuradha et al. stated that direct immunofluorescence in discoid lupus erythematosus revealed granular deposits of IgG, C3 and faint deposition of immunoglobulin M along the BMZ in 25% of cases.[15]
In our study, out of 121 psoriasis patients’ geographic tongue were observed in 21 (17.3%) patients. The major cutaneous manifestation was silvery keratotic scales. The majority of psoriatic patients fall in to 40 to 50 years of age group. However, it is impossible to conclude a direct correlation or pathogenic relationship between psoriasis and geographic tongue in the absence of genetic parameter. Our results suggest that psoriasis was observed in 121/3,500 dermatological patients in 8 months. Bedi in his study of psoriasis in 530 North Indian population suggested that the prevalence rate was 2.8% with male predilection (2.4:1). The peak age of onset was third and fourth decade.[28] Kaur et al. suggested that the prevalence rate of psoriasis in 1220 Indian population was 2.3% with male predilection.[29] Daneshpazhooh et al. conducted a case?control study on tongue lesions in 200 psoriatic patients and stated that fissured, geographic tongue were seen more frequently in psoriatic individuals than the control group. Comparatively, geographic tongue was more frequently seen than fissured tongue.[30] Hernández?Pérez et al., in his study on the prevalence of oral lesions in psoriasis, stated that oral lesions were found in 67.5% psoriasis patients. The oral lesions include fissured and geographic tongue. Furthermore, he concluded that the high prevalence of fissured tongue and geographic tongue in patients with psoriasis suggests that these lesions should be taken in account in further studies as possible predictors of the severity of the diseases.[5] Gonzaga et al. investigated the association of human leucocyte antigen (HLA) with geographic tongue and psoriasis and was highly significant. The association of HLA?CW6 with psoriasis and geographic tongue, reinforces the concept of pathogenetic relationship between geographic tongue and psoriasis.[31] Tomb et al. suggested that there was a strong correlation between psoriasis and fissured, geographic tongue. However, these features are not pathognomic for this disease. These two types of signs involving the tongue can occur in psoriasis, but the patients are generally unaware of this sign and rarely complain about it.[32]
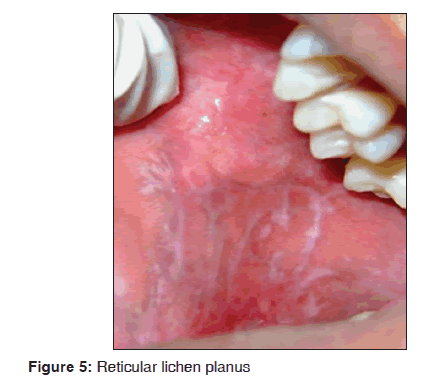
In our study, out of 72 lichen planus patients 12 (16.6%) showed oral manifestations. The age of patients fall between 30 and 40 years of age. The most common clinical type was reticular type and buccal mucosa was affected predominantly and there was a female predilection. The most common oral site was buccal mucosa as reticular type [Figure 5]. The skin lesions showed hypertophic plaque form and dystrophic nails were observed. Oral Lichen planus was characterized by the presence of hyperkeratosis, liquefactive degeneration of the basal layer and dense band of chronic inflammatory cells within connective tissue. Direct immunofluorescence demonstrated the presence of fibrinogen along the basement zone in all cases. Our results suggest that lichen planus was observed in 72/3,500 dermatological patients in 8 months. Our results were consistent with many previous studies.
Oliveira Alves et al. stated that the prevalence of oral lichen planus was 6% with female predilection.[33] Thornhill et al. mentioned histopathological features of lichen planus are characteristic but may not be specific. The other conditions such as lichenoid drug reaction, lichenoid amalgam reaction, oral graft versus host disease, lupus erythematosus, chronic ulcerative stomatits and oral mucosal cinnamon reaction may also show a similar histopathological pattern.[34] Jordan et al. stated that lichen planus shows a characteristic pattern of fibrinogen deposition along the basement zone and extending irregularly in to the superficial lamina propria described as shaggy or fibrillar pattern.[35] DeRossi and Ciarrocca stated that direct immunofluorescence of lichen planus demonstrates a ragged band of fibrinogen in the basement membrane in 100% of cases.[36] On contrary Anuradha et al. mentioned that direct immunofluorescence are not specific, further none of the oral lesions showed the characteristic pattern of staining for lichen planus specific antigen.[15]
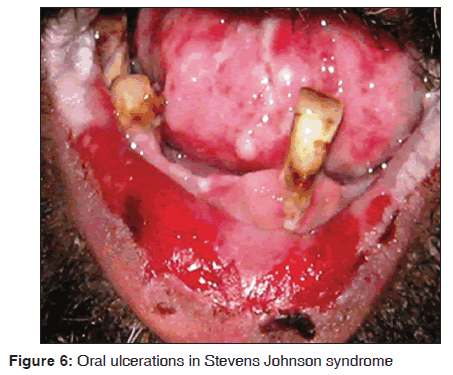
In our study, 14 of the 12 (85.7%) Stevens Johnson syndrome patients showed oral manifestations. Oral, ocular, cutaneous and genital lesions were observed in these patients. The oral manifestations were ulcerative and erosive lesions over lips, buccal mucosa, labial mucosa, soft palate, floor of mouth [Figure 6]. All the cases were diagnosed as human immunodeficiency virus (HIV) sero?positive, and under highly active anti?retroviral therapy (HAART) regimen, which includes lamivudine (150 mg), zidovudine (300 mg) and nevirapine (200 mg). All the patients developed the lesions in 4?5 weeks of drug administration. Two cases did not show any oral manifestations and this may be due to lower doses of nevirapine. However, it is impossible to conclude that nevirapine as a causative drug in three drug regimen for Stevens Johnson syndrome. However, the susceptive drug is similar to that report from previous literatures. Our results suggest that Stevens Johnson syndrome was observed in 14/3,500 dermatological patients in 8 months. Singh et al. stated that Stevens Johnson syndrome occur in 0.3% of patients taking nevirapine within the first 4?6 weeks of treatment.[37] Mason et al. stated that oral lesions were observed after 28 days of drug administration.[38] Namayanja et al. in 2005, stated that individuals with HLA BW44, HLA B12 and HLA DQB1 * 0601 appear to be more susceptible in developing this disease.[39] Balasundaram et al. stated that the cutaneous hypersensitivity reaction with extreme oral lesions were seen following the nevirapine therapy. Patients have shown elevated liver enzymes and hepatitis.[40]
In our study, three of the two toxic epidermal necrolysis patients were HIV infected and under HAART and another patient was under antimalarial drug therapy. HAART (three drugs regimen) includes lamivudine (150 mg), zidovudine (300 mg) and nevirapine (200 mg). Whereas, anti?malarial drug therapy was included quinine (100 mg). These patients showed severe extensive ulcerative and erosive areas over oral mucosa and all over the body. Patient with quinine therapy showed trismus. However, it is impossible to conclude that nevirapine as a causative drug in three drug regimen for toxic epidermal necrolysis. However, the susceptive drug is similar to that report from previous literatures. Our results suggest that toxic epidermal necrolysis was observed in 3/3,500 dermatological patients in 8 months. Cattelan et al. reported two cases of toxic epidermal necrolysis induced by nevirapine therapy. Patient presented with multiple ulcerations and erosive areas in the oral mucosa.[41] Heng stated that toxic epidermal necrolysis is a severe and often widespread bullous skin disease. Many drugs had been reported to cause toxic epidermal necrolysis, such as sulfonamides, penicillin and quinine.[42] Owotade and Greenspan stated that quinine can cause dystonia affecting eyes and limbs. Oral features include trismus, involuntary movement of tongue and deviation of the jaw and severe adverse reactions include Stevens Johnson syndrome and toxic epidermal necrolysis.[43]
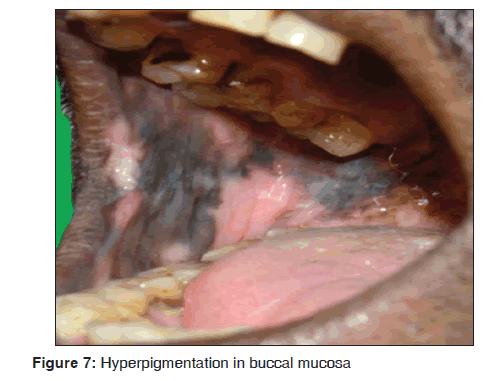
In our study, five cases showed hyperpigmentation of the oral mucosa over the palate and buccal mucosa [Figure 7] were diffuse and irregular patches, which were dark brown to brownish black in color. All the cases were under HIV infected individuals and under HAART regimen including lamivudine (150 mg), zidovudine (300 mg) and nevirapine (200 mg). Our findings were consistent with previous studies. Our results suggest that hyperpigmentation of the oral mucosa was observed in 3/3,500 dermatological patients in 8 months. Ranganathan et al., in his study on oral lesions and conditions associated with 300 HIV infected South Indian patients, reported that 68 (23%) cases showed hyperpigmentation.[44] Ficarra et al. stated that oral hyperpigmentation may occur suddenly in HIV infected individuals and has been ascribed to a number of medications including zidovudine, ketoconazole and clofazimine.[45] Ramírez et al., in his study on oral mucosal lesions in HIV infected Mexican patients stated that third most common type of oral lesions, observed was oral hyperpigmentation.[46] Smith et al. stated that oral hyperpigmentation have a statistically significant association with HIV diseases progression.[47] Ranganathan et al. in 2004, stated that the pigmented areas in patients with HIV disease undergoing HAART were unique and were different from racial pigmentation. Common sites of pigmentation were the palate and buccal mucosa. The pigmented areas were dark brown to brownish black in color and presented as diffuse or irregular patches. The intra oral pigmentation was due to increased release of alpha melanocyte stimulating hormone, which was due to deregulated release of cytokines in HIV infectious disease.[48]
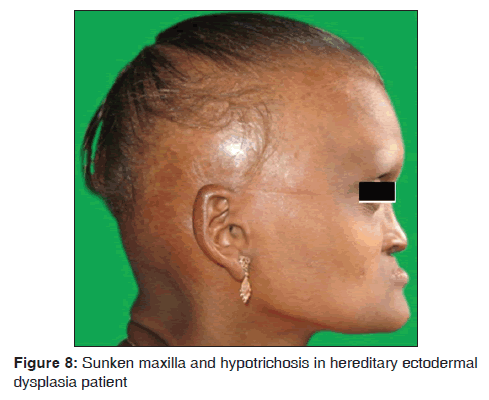
In our study, one 18?year?old female (0.02%) patient with ectodermal dysplasia was observed. She presented with complete anodontia of maxillary arch; and hypodontia of mandibular arch. The only teeth presented in the oral cavity were right and left lower second premolars [Figure 8]. Other symptoms such as anhidrosis, thin and sparse hair, dry and scaly skin, hypotrichosis, saddle nose, sunken cheeks and intolerance to heat were also observed. Our results suggest that ectodermal dysplasia was observed in 1/3,500 dermatological patients in 8 months. The findings were consistent with the previous studies. Babu et al. mentioned that the incidence of ectodermal dysplasia is 1 in 100,000 births. The features include delayed eruption of deciduous and permanent teeth, intolerance of heat and less sweat production and dry skin.[49] Murali Gopika Manoharan et al. reported a case of 10?year?old boy with hereditary ectodermal dysplasia with complete anodontia of both arches, palatal tori, absence of sweating and intolerance to heat, sparse, blond scalp hair.[50] Wright et al., the genetic studies, suggested that different genes affecting closely related components of a common molecular pathway such as Ectodysplasin-A (EDA1), Ectodysplasin-A Receptor (EDAR) and Ectodysplastin A Receptor- Associated adapter protein (EDARADD) in Nuclear factor kappa-light-chain-enhancer of activated B cells (NF kB) Pathway.[51] Bala and Pathak reported a case on hypohidrotic ectodermal dysplasia in 8?year?old male child with complete anodontia of primary as well as secondary dentition. Patient exhibited intolerance to heat, smooth and dry skin and sparse light?colored eyebrows. This hereditary condition is characterized by the absence or defect of two or more ectodermally derived structures. The most commonly observed forms of ectodermal dysplasia are the hidrotic and hypohidrotic types; discrimination is based on the presence or absence of sweat glands.[52]
Future Direction
Future studies needs to focus on sample size on individual disease, which may be useful to identify the statistical correlation between oral manifestations in dermatological diseases among the individual diseases. Although the geographic/fissured tongue is seen in psoriatic individuals the debate is continuously present while some researchers believe it to be a correlation, whereas others believe as coincidental oral finding in psoriatic disease. Genetic and Human Leukocyte Antigen association studies need to be employed, which may unravel the concepts of correlation and coincidence in the forementioned debate.
Conclusion
Even though, the number of patients with oral mucosal lesions in dermatological diseases was relatively low in this series. The striking observation in the study was the association of autoimmune diseases was higher. A special emphasize of high morbidity and mortality index with autoimmune disease process alerts the dental practice. A focus on the prevalence of such lesions in dental and dermatology practice may underline the burden of diagnosis of such conditions. The significance of diagnosing these oral lesions in dermatology practice and mucocutaneous lesions in dental practice plays a pivotal role in patient management. Thus, widespread and panoramic vision of knowledge needs to be employed in diagnosing these cases in dental and dermatology practice. Hence, multidisciplinary therapeutic approach will welcome the patient’s good prognosis.
Acknowledgments
We thank Dr. L. Krishna Prasad, Principal, SIBAR Institute of Dental Sciences for his continuous support and motivation for research activities. We extend our thank note to Dr. Rajarajeswari Kantheti, Principal, Guntur Medical College, Guntur and Department of Dermatology, Government General Hospital, Guntur, Andhra Pradesh for their support and academic drive. The study was designed in emphasis of multidisciplinary approach of dental practice and it was self?funded and no grants were obtained from any societies across the globe.
Source of Support: The research work was carried with self funding.
Conflict of Interest: None declared.
References
- Jahanbani J, Sandvik L, Lyberg T, Ahlfors E. Evaluation of oral mucosal lesions in 598 referred Iranian patients. Open Dent J 2009;3:42?7.
- Francis V Howell. Oral mucous membrane lesions: Pathologic features. California medicine 1964;100:186?91.
- Barber HW, Cantab BC. The relationship of dental infection to diseases of the skin. Sect Odontol 1927;20:1421?30.
- Ramírez?Amador VA, Esquivel?Pedraza L, Orozco?Topete R. Frequency of oral conditions in a dermatology clinic. Int J Dermatol 2000;39:501?5.
- Hernández?Pérez F, Jaimes?Aveldañez A, Urquizo?Ruvalcaba Mde L, Díaz?Barcelot M, Irigoyen?Camacho ME, Vega?Memije ME, et al. Prevalence of oral lesions in patients with psoriasis. Med Oral Patol Oral Cir Bucal 2008;13:E703?8.
- Burket LW, Greenberg MS, Glick M. Burket’s Book of Oral Medicine. 10th ed. India: Elsevier Publications; 2008. p. 42?8.
- Mohan KH, Pai S, Rao R, Sripathi H, Prabhu S. Techniques of immunofluorescence and their significance. Indian J Dermatol Venereol Leprol 2008;74:415?9.
- Goncalves LM, Bezerra Junior JR, Cruz MC.Clinical evaluation of oral lesions associated with dermatologic diseases. An Bras Dermatol 2010;85:150?6.
- Suliman NM, Astrøm AN, Ali RW, Salman H, Johannessen AC. Oral mucosal lesions in skin diseased patients attending a dermatologic clinic: A cross?sectional study in Sudan. BMC Oral Health 2011;11:24.
- Progress in Autoimmune Diseases Research. The Autoimmune Diseases Coordinating Committee. Report to Congress. National Institutes of Health. National Institute of Allergy and Infectious Disease. United States: US Department of Health and Human Services National Institute of Health; 2005. p. 16?24.
- Libu GK, Bina T, Raphael L, Balakrishnan SE, George B, Samson JF. Prevalence and socio demographic determinants of skin disease among lower primary school children in Calicut, Kerala. Kerala Med J 2009;5:185?90.
- Kumar KA. Incidence of pemphigus in Thrissur district, South India. Indian J Dermatol Venereol Leprol 2008;74:349?51.
- Shamim T, Varghese VI, Shameena PM, Sudha S. Pemphigus vulgaris in oral cavity: Clinical analysis of 71 cases. Med Oral Patol Oral Cir Bucal 2008;13:E622?6.
- Stanley JR, Koulu L, Thivolet C. Distinction between epidermal antigens binding pemphigus vulgaris and pemphigus foliaceus autoantibodies. J Clin Invest 1984;74:313?20.
- Anuradha Ch, Malathi N, Anandan S, Magesh K. Current concepts of immunofluorescence in oral mucocutaneous diseases. J Oral Maxillofac Pathol 2011;15:261?6.
- Adam BA. Bullous diseases in Malaysia: Epidemiology and natural history. Int J Dermatol 1992;31:42?5.
- Gudi VS, White MI, Cruickshank N, Herriot R, Edwards SL, Nimmo F. Annual incidence and mortality of bullous pemphigoid in the Grampian region of North?East Scotland. Br J Dermatol 2004;122:1091?5.
- Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris – Incidence and mortality in the UK: Population based cohort study. BMJ 2008;337:a180.
- Ata?Ali F, Ata?Ali J. Pemphigus vulgaris and mucous membrane pemphigoid: Update on etiopathogenesis, oral manifestations and management. J Clin Exp Dent 2011;3:246?50.
- Challacombe SJ, Setterfield J, Shirlaw P, Harman K, Scully C, Black MM. Immunodiagnosis of pemphigus and mucous membrane pemphigoid. Acta Odontol Scand 2001;59:226?34.
- Mutasim DF, Anhalt GJ, Diaz LA, Patel HP. Linear immunofluorescence staining of the cutaneous basement membrane zone produced by pemphigoid antibodies: The result of hemidesmosome staining. J Am Acad Dermatol 1987;16:75?82.
- Malaviya AN, Singh RR, Singh YN, Kapoor SK, Kumar A. Prevalence of systemic lupus erythematosus in India. Lupus 1993;2:115?8.
- Yacoub Wasef SZ. Gender differences in systemic lupus erythematosus. Gend Med 2004;1:12?7.
- Jayakumar ND, Jaiganesh R, Padmalatha O, Sheeja V. Systemic lupus erythematosus. Indian J Dent Res 2006;17:91?3.
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725.
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003;349:1526?33.
- Pongsiriwet S, Kuntharaniranan T, Thosaporn W. Oral discoid lupus erythematosus. Chiang Mai Dent J 2010;31:21?31.
- Bedi TR. Psoriasis in North India. Geographical variations. Dermatologica 1977;155:310?4.
- Kaur I, Kumar B, Sharma VK, Kaur S. Epidemiology of psoriasis in a clinic from north India. Indian J Dermatol Venereol Leprol 1986;52:208?12.
- Daneshpazhooh M, Moslehi H, Akhyani M, Etesami M. Tongue lesions in psoriasis: A controlled study. BMC Dermatol 2004;4:16.
- Gonzaga HF, Torres EA, Alchorne MM, Gerbase?Delima M. Both psoriasis and benign migratory glossitis are associated with HLA?Cw6. Br J Dermatol 1996;135:368?70.
- Tomb R, Hajj H, Nehme E. Oral lesions in psoriasis. Ann Dermatol Venereol 2010;137:695?702.
- Oliveira Alves MG, Almeida JD, Balducci I, Guimarães Cabral LA. Oral lichen planus: A retrospective study of 110 Brazilian patients. BMC Res Notes 2010;3:157.
- Thornhill MH, Sankar V, Xu XJ, Barrett AW, High AS, Odell EW, et al. The role of histopathological characteristics in distinguishing amalgam?associated oral lichenoid reactions and oral lichen planus. J Oral Pathol Med 2006;35:233?40.
- Jordan RC, Daniels TE, Greenspan JS, Regezi JA. Advanced diagnostic methods in oral and maxillofacial pathology. Part II: Immunohistochemical and immunofluorescent methods. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;93:56?74.
- DeRossi SS, Ciarrocca KN. Lichen planus, lichenoid drug reactions, and lichenoid mucositis. Dent Clin North Am 2005;49:77?89.
- Singh H, Kachhap VK, Kumar BN, Nayak K. Nevirapine induced Stevens?Johnson syndrome in an HIV infected patient. Indian J Pharmacol 2011;43:84?6.
- Mason AR, Cortes GY, Pollack RB. Nevirapine?induced Stevens?Johnson syndrome in a pediatric patient. Pediatr Dermatol 2008;25:128?9.
- Namayanja GK, Nankya JM, Byamugisha JK, Ssali FN, Kityo CM, Rwambuya SD, et al. Stevens?Johnson syndrome due to nevirapine. Afr Health Sci 2005;5:338?40.
- Balasundaram S, Ranganathan K, Umadevi K, Gunaseelan R, Kumaraswamy N, Solomon S, et al. Oral lesions associated with nevirapine?related Stevens Johnson syndrome: A report of four cases. J Oral Maxillofac Pathol 2011;15:39?45.
- Cattelan AM, Trevenzoli M, Sasset L, Sgarabotto D, Lanzafame M, Meneghetti F. Toxic epidermal necrolysis induced by nevirapine therapy: Description of two cases and review of the literature. J Infect 2001;43:246?9.
- Heng MC. Drug?induced toxic epidermal necrolysis. Br J Dermatol 1985;113:597?600.
- Owotade FJ, Greenspan JS. Malaria and oral health. Oral Dis 2008;14:302?7.
- Ranganathan K, Reddy BV, Kumarasamy N, Solomon S, Viswanathan R, Johnson NW. Oral lesions and conditions associated with human immunodeficiency virus infection in 300 south Indian patients. Oral Dis 2000;6:152?7.
- Ficarra G, Shillitoe EJ, Adler?Storthz K, Gaglioti D, Di Pietro M, Riccardi R, et al. Oral melanotic macules in patients infected with human immunodeficiency virus. Oral Surg Oral Med Oral Pathol 1990;70:748?55.
- Ramírez V, González A, de la Rosa E, González M, Rivera I, Hernández C, et al. Oral lesions in Mexican HIV?infected patients. J Oral Pathol Med 1990;19:482?5.
- Smith KJ, Skelton HG, Yeager J, Ledsky R, McCarthy W, Baxter D, et al. Cutaneous findings in HIV?1?positive patients: A 42?month prospective study. Military medical Consortium for the advancement of retroviral research (MMCARR). J Am Acad Dermatol 1994;31:746?54.
- Ranganathan K, Umadevi M, Saraswathi TR, Kumarasamy N, Solomon S, Johnson N. Oral lesions and conditions associated with human immunodeficiency virus infection in 1000 South Indian patients. Ann Acad Med Singapore 2004;33:37?42.
- Babu SG, Castelino RL, Shetty SR, Rao KA. Hereditary ectodermal dysplasia: A case report. Dentistry 2011;2:2?7.
- Murali Gopika Manoharan GV, Sabarigirnathan C, Egammai VS. Hereditary anhidrotic ectodermal dysplasia with torus palatinus?A case report. J Indian Dent Assoc 2011;5:718?21.
- Wright JT, Morris C, Clements SE, D’Souza R, Gaide O, Mikkola M, et al. Classifying ectodermal dysplasias: Incorporating the molecular basis and pathways (Workshop II). Am J Med Genet A 2009;149A: 2062?7.
- Bala M, Pathak A. Ectodermal dysplasia with true anodontia. J Oral Maxillofac Pathol 2011;15:244?6.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.