A Systematic Review of the Literature to Assess Self-medication Practices
2 Hannover Medical School, Hannover, Germany, Email: dnyanesh.limaye@hs-hannover.de
3 Faculty III, Hochschule Hannover, University of Applied Sciences and Arts, Germany, Email: dnyanesh.limaye@hs-hannover.de
Citation: Dnyanesh Limaye. A Systematic Review of the Literature to Assess Self-medication Practices. Ann Med Health Sci Res. 2017; 7: 1-15
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Self-medication, practiced globally is an important public health problem. Research studies have indicated inappropriate self-medication results in adverse drug reactions, disease masking, and inaccurate diagnosis of disease, increased morbidity, drug interactions, antibiotic resistance and wastage of healthcare resources. Rationale: Several self-medication systematic reviews have been written for specific population like adolescents, elderly population and medical students, etc. or for particular country or for specific disease. As the issue of self-medication and associated antibiotic resistance presents an important global public health problem with a reported worldwide increase in self-medication practices in the last decade, an integration of available global self-medication data could be useful to get a comprehensive yet comparative understanding about associated problems, antibiotic resistance, reaching stronger conclusions and planning future intervention than those drawn from reviews on specific target diseases or populations. We therefore intended to systematically review the existing literature on global self-medication practices in order to gain comprehensive information regarding prevalence, sample populations, medications used, target disease and reasons for self-medication. Methods: We conducted a systematic review of the literature on self-medication practices by searching, PubMed Medline and Web of Science database using the following combination of keywords - self-medication, self-prescription, non-prescription. Truncation was used to ensure retrieval of all possible variations of search terms. The search was limited to articles published between 1st January 2000 and 31st December 2016, human studies and English language. Duplicate and irrelevant studies were excluded from the final review. Results: A total of 140 studies covering 189279 subjects from children, adults, to geriatric population, with the overall prevalence of SM ranging from 0.1% to 100%, were included in the review. Studies were from diverse geographical locations. Majority of the studies were from Brazil (12; 9%), followed by 9 (6%) each from India (9; 6%) and Pakistan and 8 (6%) from Nigeria. Most of the studies (44; 31%) were done to find antibiotic SM prevalence, and 75 (54%) studies were done without any specific therapy area focus. Antibiotics (82; 59%), followed by NSAID’s (43.31%), and cough and cold medicines (13.9%) were the most widely self-medicated drugs. Cough and cold (61.43%), body pain (45; 32%), gastrointestinal complaints (44. 31%) were the top reported health complaints in the studies and pharmacy (80.57%), relatives & friends (45.32%), left overs (31; 22%), were the most common sources of self-medication. Conclusion: Self-medication was seen as a widespread phenomenon along with antibiotics as most widely self- medicated drugs. The World Health Organization reported alarming levels of resistance to antibiotics in member countries. The misuse of antibiotics poses a serious risk to infectious disease control and public health in general. Lack of knowledge in lay people and lack of attention of the public health researchers regarding not only self-medication but associated important problem like antibiotic resistance and potential adverse events deserves immediate implementation public health programs for increasing awareness and importance of this issue. At the same time, while implementing the rules and regulations governments should improve on providing adequate and affordable access to health care services. Further cross sectional studies with a standardized methodological approach are needed to gain better comparative understanding of global SM prevalence and practices.
Keywords
Self-medication; Self-prescription; Non-prescription; Antibiotic resistance
Introduction
Quest for recovery from diseases and having wellness is a natural health seeking behaviour. In response, self-medication is the most commonly seen phenomenon [1]. The term self-medication (SM) refers to the use of drugs to treat self-diagnosed disorders or symptoms, or the intermittent or continued use of a prescribed drug for chronic or recurrent disease or symptoms [2,3]. Main sources of self-medication are relatives, friends, pharmacists who not only provide medicines but also the information about drug’s use [4,5]. At the same time several factors like lack of access to health care, physician fees, time constraint, lack of trust on physician, inadequate implementation of drug laws have been shown to influence self-medication behavior [5-7].
Self- medication is an important public health problem and is practiced globally [3,7-9]. with a reported prevalence of 21% in Lithuania [10]. 19.8% in Romania [10]. 15.2% in Spain [10]. 21% in Portugal [8]. 31% in Czech Republic [11] among European countries. In developing countries reported prevalence rates are much higher, with 79% in India [12]. 84% in Pakistan [3]. 78% in Saudi Arabia [13]. 67% in Nigeria [14].
Several studies have indicated that inappropriate self-medication results in adverse drug reactions, disease masking, inaccurate diagnosis of disease, increased morbidity, drug interactions, antibiotic resistance and wastage of healthcare resources [7,15-18].
Rationale
In the past several years, several systematic reviews on self medication have been written for specific population like adolescents [5,19] elderly population [20] physician and medical students [21] for particular countries [22,23]. or for specific disease [24-26]. As self-medication has a substantial impact on public health with growing practice [27-29]. it is helpful to have the issue of selfmedication presents an important public health issue, it may not only be a problem but could also offer advantages in many situations. There has been reported increase in self-medication practices in the last decade worldwide [27-29]. As self-medication has a substantial impact on public health with growing practice, it is helpful to integrate global self-medication data. It would give comprehensive yet comparative understanding, reaching stronger conclusions and planning future intervention than those drawn from reviews on specific target diseases or populations. As a part of the background for this effort, existing literature on self-medication was reviewed. This review provides an overview of global self-medication practices. It gives comprehensive information regarding prevalence, sample populations, medications used, target disease and reasons for self-medication.
Methods
Search strategy
Authors conducted a systematic review of the literature on selfmedication practices. Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram and guidance set out by the Centre for Reviews & Dissemination was followed for this review [30,31]. understanding of diseases and health, growth in information technology, number of available medications, and drug information have been identified as key factors contributing to increase in self-medication practices in the last decade [27-29]. Based on this, PubMed Medline and Web of Science database were searched for papers published between 1st January 2000 and 31st December 2016. Title/ abstract search was conducted with a combination of key words: self-medication, self-prescription, non-prescription. Truncation was used to ensure retrieval of all possible variations of search terms. As per Centre for Review and Dissemination (CRD) Guidance a search strategy for PubMed Medline and Web of Sciences is presented here in Figure 1 [30].
Screening and eligibility criteria
Pairs of reviewers (four - eyes principle) - independently screened the resultant literature from PubMed and Web of Science by reviewing their titles and abstracts. Search was limited to articles in English language and available as full text publication. Eligible studies for full text review were selected by excluding duplicate studies. Studies which were opinions, critiques of previous studies, letters to editors, were excluded and only those explicitly referring to self-medication practices were included for full text review. Two reviewers appraised the full text of each study independently. Any discrepancies between two reviewers were resolved through discussion, or involving a third reviewer as arbiter, if necessary. Finally, a third reviewer - checked all the excluded and included studies to validate the final selection of publications. Limiting the search to these two databases may not provide access to all self-medication studies and may influence the results, authors are aware of this as a study limitation.
Data extraction
Two reviewers screened all the full text articles to look for selfmedication practices details. Based on the results, a database was designed to enlist the self-mediation components addressed by them. Following self-medication component details were extracted from each study: year of publication, country of origin, population sampled, self-medication therapy area if any, selfmedication definition, study objective, socioeconomic details, health insurance, reasons, sources, medical conditions treated, antibiotic resistance awareness, patient leaflet use, adverse events checks, place and survey method, recall period, ethical approval for study, and pilot study information.
Results
Literature search results
The literature search as shown in Figure 1 generated a total 1663 studies from PubMed Medline and Web of Science. Title and abstract review was done to remove duplicate (342) and irrelevant studies (1181). (We defined irrelevant studies as – publications not based on own studies conducted by authors to investigate self-medication or which are giving opinion or critique of previous studies, or with a focus on drug dependency, substance abuse, safety database, pharmacist views, mood disorders, medical ethics and emotional socialization). Finally 140 studies were included in the systematic review. The study details are presented in Table 1 [32-154].
| Country | Sample size | Study Therapy Area | Self-medication % |
|---|---|---|---|
| Argentina (2)[100,101] | 176-379 | Ophthalmology, Psoriasis | 33-100 |
| Bahrain (2)[15,17] | 134-141 | NS | 45-73 |
| Bangladesh (2)[18,116] | 500-1300 | Antibiotics, NS | 26-100 |
| Brazil (12)[52,58,68,69,80,85,89,98,105,109,120,123] | 772 | NS | 24-86 |
| Cameroon (1) [1] | 283 | Dental | 67 |
| China (6) [32,86,90,112,130,149] | 30-1459 | Antibiotics, NS | 40-82 |
| Colombia (1) [125] | 414 | NS | 77 |
| Croatia (2) [ 41,111] | 287-389 | Analgesic, NS | 74-88 |
| Czech Republic (1) [11] | 979 | NS | 75 |
| Congo (1) [127] | 251 | NS | 54 |
| Denmark (1) [44] | 1959 | Antibiotics | 3 |
| Egypt (1) [147] | 1100 | NS | 86 |
| Ehiopia (4) [17,148,121,133] | 270-1257 | Antibiotics, NS | 18-100 |
| Euro-Mediterranean region (1) [66] | 1705 | Antibiotics | 01 to 37 |
| Europe (4) [9,10,49,55] | 1090-15548 | Antibiotics | 0.1 to 62 |
| Finland (1) [42] | 857 | NS | 65 |
| France (2) [107,155] | 100-3027 | Steroids, NS | 15-84 |
| Ghana (1) [91] | 600 | Antibiotics | 70 |
| Greece (2) [73,128] | 150-1139 | Antibiotics, NS | 54,77 |
| Guatemala (1) [134] | 418 | Antibiotics | 78 |
| India (9) [6,12,54,87,99,139,146,151,153] | 100-1928 | Dentistry, NS | 18-78 |
| Indonesia (1) [135] | 25 | Antibiotics | 100 |
| Iran (6) [7,47,73,106,129,140] | 180-500 | Migraine, Antibiotics | 35-91 |
| Israel (1) [38] | 467 | Antibiotics | 10 |
| Italy (3) [93,102,141] | 1269 | Antibiotics, NS | 23-69 |
| Jordan (4) [60,63,144,152] | 819 | Antibiotics, NS | 40-87 |
| Kuwait (3) [43,56,136] | 100-1110 | Diabetes, Antibiotics, NS | 13-92 |
| Lao (1) [45] | 500 | Antibiotics | 16 |
| Lebanon (1) [114] | 319 | Antibiotics | 42 |
| Lithuania (1) [138] | 1005 | Antiobiotics | 31 |
| Malaysia (1) [29] | 314 | NS | 67 |
| Mexico (1) [70] | 245 | NS | 53 |
| Nepal (1) [35] | 142 | NS | 59 |
| Nigeria (8) [14,40,50,75,81,82,83,93] | 210-4128 | Antibiotics, Malaria, Colic, Menstrual Disease, Dentistry, ENT, Antimalarial, NS | 24-91 |
| Pakistan (9) [3,59,84,96,108,113,122,125,150] | 150-1850 | Antibiotics, NS | 15-95 |
| Palestine (2) [61,104] | 565-1581 | NS | 87-98 |
| Poland (2) [103,137] | 609-891 | Antibiotics | 11-41 |
| Portugal (3) [8,48,117] | 1192-3198 | Antibiotics, NS | 21-39 |
| Romania (1) [110] | 461 | NSAID | 80 |
| Saudi Arabia (3) [13,131,142] | 504 | NSAID, Antibiotics | 49-75 |
| Serbia (2) [124,143] | 383-1296 | NS | 46-79 |
| Slovenia (4) [77,78,79,88] | 410-1294 | NS | 38-92 |
| Spain (4) [34,46,76,95] | 190-20738 | Antibiotics, NS | 20-77 |
| Sri Lanka (1) [132] | 175 | Antibiotics | 100 |
| Sudan (3) [16,39,51] | 1000-1750 | Antibiotics And Anti-malarials | 73-82 |
| Sweden (1) [37] | 690 | Antibiotics | 0.3 |
| Tanzania (1) [119] | 93 | Malaria | NS |
| Thailand (2) [53,71] | 400-3720 | NS | 86-92 |
| Turkey (4) [33,36,64,154] | 602-3521 | Antibiotics, NS | 19-58 |
| UAE (1) [67] | 860 | Antibiotics | 56 |
| Uganda (1) [115] | 884 | Antibiotics | 75 |
| USA (3) [62,65,74,97] | 19-691 | Antibiotics, NS | 31-100 |
| Vietnam (1) [145] | 160 | NS | 39 |
| Yemen, Saudi Arabia, and Uzbekistan (1) [118] | 1200 | Antibiotics | 78 |
NS=Non-specific; ENT=Ear, Nose, Throat, NSAID= Non-steroidal anti-inflammatory drugs
Table 1: Self-Medication prevalence from various countries.
Study characteristics
The studies in the 140 publications differed in sample size, therapy areas, self-medication prevalence, recall period, and location. These studies covered 189279 subjects from children, adults, to geriatric population. Sample size ranged from 19 to 20738. The articles reviewing self-medication prevalence rates originated from different countries (Table 1).
Six studies were multinational in nature and investigated crossnational prevalence of SM among adults from Yemen, Saudi Arabia, and Uzbekistan [118]. Euro-Mediterranean - Cyprus, Egypt, Jordan, Lebanon, Libya, Tunisia and Turkey [66] and Europe. Majority of the studies were from Brazil (12; 9%) [52,58,68,69,80,85,89,98,105,109,120,123]. followed by 9 (6%) each from India (9; 6%) [6,12,54,87,99,139,146,151,153]. and Pakistan [3,59,84,96,108,113,122,12 5,150]. and 8 (6%) from Nigeria.
Majority of the studies used face to face interview method (98.70%) for data collection, while self-administered questionnaire technique was used for remaining 42 (30%) studies. Seventy-five (54) studies were done without any focus on specific therapy area for self-medication, whereas 44 (31%) studies investigated self-medication prevalence of antibiotics, rest of the studies were in other therapy areas for example: malaria, analgesic, diabetes, menstrual symptoms etc.
Results based on specific self-medication aspects
Definition of self-medication: The phrasing of self-medication definition was inconsistent across studies. Twenty-six (18%) studies failed to report definition of SM. However the most common themes used in definition were as follows: use of drugs without consultation of healthcare professional (57; 40%), use of drugs without prescription (27; 19%), use of the drugs with own initiative (24; 17%). Remaining 8 (6%) studies used varied definitions of self-medication such as – using the drug with lay advice, at the recommendation of family/friends, use after demanding from pharmacist etc.
Performance characteristics of SM: After a review of 140 publications, it was seen that only 93(66%) publications reported that they had obtained ethical approval for conducting the research study. Reported recall period varied from - on that day to ever in life. Before conducing the main study only 63(45%) publications reported piloting before initiation of the study. Response rates varied from as low as 2% for a web-based platform study and 18% to as high as 100% in face to face interview studies (Table 2) [155-172].
| Variables | Range |
|---|---|
| Overall prevalence of SM | 0.1% to 100 % |
| Antibiotic SM prevalence | 1 to 100% |
| Sample size | 19 to 20738 |
| Recall period | point of time to lifetime use |
| Response rate | 2 % to 100 % |
| Total participant population | 189279 |
| Participants self-medicated | 81665 (43%) |
| Variables | Number of studies (%) |
| Ethical approval | 91 (65 %) |
| Pilot study | 63 (45 %) |
| Income | 51 (36%) |
| Education | 115 (82%) |
| Health insurance | 28 (20%) |
| Antibiotic resistance | 12 (9%) |
| Prescribing information reading | 18 (13%) |
| Adverse event awareness | 49 (35%) |
| Drugs used for self-medication | |
| • Antibiotics [1,3,6,7,8,9,10,12,32,33,34,36,37,38,39,41,44,45,46,48,49,50,51,52,54,55,57,58,59,60,61,66,67,69,70,72,73,74,75,82,85,88,89,93,94,96,97,100,103,104,105,114,115,116,117,118,119,122,123,124,125,126,130,132,134,135,136,138,139,140,141,142,143,144,148,149,150,151,152,13,154,156] | 82 (59%) |
| • NSAID [3,6,7,12,15,33,35,41,47,53,57,58,59,60,61,68,69,70,72,82,84,85,89,96,97,100,105,111,122,123,124,125,126,127,132,140,141,142,143,144,145,148,154] | 43 (31%) |
| • Cough & cold medications [7,8,12,85,57,61,68,100,105,125,130,154,156] | 13 (9%) |
| • Antimalarial [39,40,51,94,120] | 5 (4%) |
| Sources of self-medication | |
| • Pharmacy[1,3,7,9,10,11,13,17,18,29,32,33,34,35,36,39,43,45,46,47,50,51,52,53,54,55,56,59,60,63,64,65,66,71,73,76,78,79,80,83,84,85,86,89,90,91,94,97,99,101,103,104,105,109,113,115,116,119,120,122,123,124,126,134,135,137,138,139,140,143,144,145,147,148,149,150,151,153,154,157] | 80 (57%) |
| • friends and relatives [1,3,9,10,17,39,44,45,51,52,53,54,55,56,59,61,63,64,65,66,73,74,78,79,80,83,84,89,90,91,94,104,119,120,126,137,138,139,140,147,150,151,153,154,156] | 45 (32%) |
| • left overs [6,9,10,32,39,44,52,64,67,73,84,94,99,104,113,116,118,120,130,135,137,138,139,143,144,147,148, 149,150,154,156] | 31 (22%) |
| • home pharmacy [6,7,11,18,41,59,66,103,120,125,126] | 11 (8%) |
| • previous prescriptions [6,17,54,55,63,90,119,124] | 8 (6%) |
| Reasons for self-medication | |
| • time constraint [1,3,6,11,12,13,15,17,29,34,39,50,53,54,55,57,59,60,63,64,66,78,83,84,87,90,92,94,98,99,100,101,103,105,107,109,113,114,115,116,117,118,120,122,126,127,131,132,134,135,136,141,143,147,149,150,153,154,157] | 59 (42%) |
| • cost constraint [1,3,6,13,15,17,29,35,39,50,51,53,54,60,61,62,64,65,66,69,82,83,84,87,91,92,98,99,100,101,102,105,107,109,113,114,115,117,119,120,122,126,127,132,135,136,141,146,149,150,151,152,153] | 53 (38%) |
| • mild disease [1,6,12,13,15,35,57,59,60,61,83,100,105,109,122,126,127,132,135,141,142,143,145,146,147,148,150,153] | 28 (20%) |
| • Have knowledge [12,14,18,29,32,35,40,53,55,63,69,74,97,99,101,107,116,118,127,139,140,141,152,153,154] | 25 (18%) |
| • past experience [3,6,11,13,29,39,51,57,59,63,64,65,66,71,74,105,114,117,122,132,136,145,149,150] | 24 (17%) |
| • no trust in physician [6,47,60,61,78,87,102,105,117,126,134,135,141,146,151,157] | 16 (11%) |
| • convenience [13,59,63,71,91,113,135,151,152] | 9 (6%)) |
| • previous prescription [12,92,109,126,129,142,148] | 7 (5%) |
| Self-medicated health complaints | |
| • Cough and cold [6,8,12,13,15,17,29,32,33,34,39,42,43,44,46,48,51,52,55,57,58,59,61,63,64,65,66,69,73,77,78,84,88,91,94,97,100,103,105,109,111,114,117,119,120,125,126,127,128,132,133,135,137,139,141,147,149,151,152,154,156] | 61 (43%) |
| • Body pain [1,3,8,10,12,29,34,35,42,43,48,53,54,58,61,62,69,70,71,72,78,83,84,85,90,97,99,100,105,109,111,120,124,125,126,129,132,135,141,143,144,145,148,149,155] | 45 (32%) |
| • GI diseases [3,8,13,14,17,29,32,33,45,48,52,53,61,65,69,70,76,84,88,94,97,100,114,115,116,117,119,120,122,123,127,128,129,133,134,135,137,141,144,146,147,148,155,157] | 44 (31%) |
| • Fever[3,6,12,17,33,34,35,52,54,58,59,64,66,67,71,73,77,84,85,91,97,99,100,103,109,116,119,120,123,125,133,135,139,143,147,149,152,154,155,156] | 40 (29%) |
| • Infection [8,9,10,12,13,36,37,39,44,51,63,64,65,66,67,70,74,85,101,105,109,114,115,125,140,141,143,144,146,148,153,155] | 32 (23%) |
| • Headache [3,10,15,17,33,35,42,47,52,54,59,76,111,112,116,122,123,124,126,129,132,147,149,154] | 24 (17%) |
| • Menstrual problems [6,43,45,53,57,76,99,112,119,124,132] | 11 (8%) |
| • Skin disease [13,32,43,53,71,74,76,115,129,133] | 10 (7%) |
| • Malaria [39,40,51,94,120,128] | 6 (4%) |
Table 2: Summary of results.
Prevalence rates and associated factors
The overall prevalence of SM ranged from 0.1% to 100% and 3 studies did not report SM prevalence (Table 2) [62,86,119]. Thirtyseven studies had low (less than 36% i.e. below 25th percentile), 63 studies had medium (between 36% to 77% i.e., 25th to 75th percentile), whereas 40 studies had high prevalence (more than 77% i.e., above 75th percentile) of SM.
Most of the studies (44.31%) were done to find antibiotic SM prevalence, some other therapy areas studied were analgesics, malaria, and psoriasis etc., whereas 75 (54%) studies were done without any specific therapy area focus.
Antibiotics SM prevalence varied from 1% to 100%. Seventeen studies had low, 21 studies had medium, whereas 7 studies had high prevalence of SM. High antibiotic prevalence was reported from studies done in the developing countries like Indonesia, Sri Lanka, Nigeria, Sudan, Guatemala etc. Greece was the only country belonging to developed nations which showed a high antibiotic SM prevalence 77% [72].
A cross national study done in 19 European countries showed high prevalence rates of self-medication with antimicrobial drugs in eastern and southern Europe and low in northern and Western Europe [10]. Younger age, higher education, and presence of a chronic disease were associated with higher rates of self-medication. Another cross national study done in 12 European countries indicated that higher gross domestic product (wealth) and exact dispensation of prescribed tablet quantities by pharmacies were independently associated with lower likelihood of antibiotic self-medication [55]. A study in Euro-Mediterranean region revealed that prevalence of selfmedication with antibiotics varied from <0.1% in Cyprus to 37% in Lebanon . Rates of self-medication rose with increasing age in Lebanon. A study done in Yemen, Saudi Arabia, and Uzbekistan documented self-medication prevalence of antibiotics varied from 48% in Saudi Arabia to 78% in Yemen and Uzbekistan [148]. In Yemen and Saudi Arabia younger women and respondents with a lower level of education were more likely to self-medicate with antibiotics [118]. A descriptive, crosssectional study reported SM prevalence of 20% among 20738 Spanish adult populations [76]. The prevalence of self-medication was 17% for women and 14% for men (p<0.05). The variables that were independently and significantly associated with a greater probability of self-medicated consumption in women were: lower age; consumption of alcohol; smoking habit. Among men, self-medication was associated with nationality (immigrants were more likely to self-medicate), income, and alcohol consumption . A cross-sectional study done with 3,720 undergraduate students in the Northern part of Thailand showed that 86% participants used self-medication. Females had significantly higher SM prevalence than males [53].
Socioeconomics and health insurance
Income, education and health insurance aspects were looked into 51(36%), 115(82%), and 28(20%) studies respectively. Participants with higher income and higher education level and having insurance [32,63,64]. were reported to be more likely to use SM.
Antibiotic resistance, prescribing information, adverse events
Knowledge about antibiotic resistance, prescribing information reading, and adverse events awareness among participants were reported in 12(9%), 18(13%), and 49(35%) studies respectively. On the backdrop of overall lack of attention of the public health researchers on the important problems related to self-medication, concerns about antimicrobial resistance, and potential adverse effects were reported as disadvantages of self-medication with antibiotics in the urban population of Yogyakarta, Indonesia [135].
Lack of antibiotic resistance knowledge was seen among participants from Kuwait, over half of participants did not agree on the statements: ‘the unnecessarily use of antibiotics can increase the resistance of bacteria to them’ (51%), ‘resistance to antibiotics is a worldwide problem’ (53%) [136].
Another study among non-medical students from Karachi, Pakistan reported that while 63% participants denied having any knowledge about antibiotic resistance, only 20% correctly knew that indiscriminate use of antibiotics can lead to increased antibiotic resistance [113]. A study from Central Saudi Arabia investigating attitudes towards SM found that 44% participants indicated that they always read the medication pamphlet, and 29% believed that buying medication without prescription is not safe practice [142]. A study evaluating the knowledge, attitude and practice of SM among medical students from Arabian Gulf University, Bahrain found that 45% of the participants practiced SM, 72% read the package insert, and 33% reported risk of adverse reactions as a reason against SM [15].
Self-medicated drugs
The most widely self-medicated drugs were antibiotics (82; 59%), followed by NSAID’s (43; 31%), and cough and cold medicines (13; 9%). 5(4%) Studies done in Sudan. Nigeria [40,93]. and Tanzania [119]. reported SM of antimalarial drugs as shown in Table 2.
Antibiotics
Antibiotic SM varied from 1 to 100% as shown in Table 2.
Penicillins were the most commonly used medicines for SM and participants of younger age, higher education, and having a chronic disease were associated with higher prevalence of self-medication. Authors also reported that, respondents’ self-diagnosed disorders were self-limiting and antimicrobial drugs would not have been indicated [10]. Seventy percent of the university students from Accra, Ghana indicated that they practiced SM with antibiotics. The most commonly used antibiotic was amoxicillin (23.9%). Thirty five percent of the students who practiced self-medication indicated that their treatments were not successful, and among these 77% continued with SM but changed the antibiotic. Forty six percent of the students did not complete the full course of antibiotics, while 51% of the respondents were aware that SM could cause adverse health effects such as antibiotic resistance [91].
A study done in Bahir Dar City, Ethiopia reported 18% prevalence of SM with amoxicillin (64.4%) as most commonly used antibiotic. Individuals with lower educational status, younger age groups, and those unable to read and write were more likely to use SM with antibiotics. Authors also reported that in Ethiopia, amoxicillin is the most commonly prescribed antibiotic from which its common use in community could have arisen . Twenty six percent antibiotic SM prevalence was reported among participants from Bangladesh and metronidazole (50%) was the most frequently self-medicated antibiotic. Diarrhea, dysentery and food poisoning were the main indications for self-medication, revealing the poor hygienic food intake of the Bangladeshi people as pointed out in the study. Forty one percent participants reported excellent disease recovery and of them 7% reported side effects for the SM of antibiotics.
NSAIDs
Followed by antibiotics (82; 58%), NSAIDs (43; 30%) were the second most self-medicated drugs, as shown in Table 2. A study done to find influence of medical training on self-medication by students in Bahrain showed NSAIDs (81%) were the most commonly used drugs for SM. This correlated well with high incidence of headache, mostly stress-related among medical students as a very common indication for self-medication [57]. Similarly a study done in Tabriz, Iran showed NSAIDs (21%) were the most commonly used drugs for SM among the clients of drug stores. Administrative employees consistently requested fewer SM drugs than blue-collar workers [7]. A study done to investigate the contents of home medicine chests and their relationship with self-medication in children and adolescents in Brazil, showed that NSAIDs (27%) were most commonly stored drugs. Many of these had past their expiry date (12%) and were missing (33%) their original patient information leaflets or labels. Medicines were stored in kitchen or bathroom, which could lead to physical or chemical changes due to exposure to heat, cold, humidity and sunlight, in addition to the risk of contamination by chemical and sanitary products. Furthermore, in this study it was observed that storing medicines in the bathroom was a risk factor for self-medication, perhaps because the products are more easily accessed and more likely to be noticed there [58]. A study in dental patients from Karnataka, India showed that NSAIDs (42.5%) were the most commonly used drugs for SM with a high prevalence among females. This may be due to lower threshold towards pain in females and greater fear of dental treatments among them [139]. A study in Romania did not find significant difference in self-medication with NSAIDs among urban (92%) and rural (81%) participants. There were no significant differences based on education, or gender among the participants [110].
Anti-malarials
Antimalarial self-medication prevalence varied from 4.4% to 50%. A study done in Khartoum, Sudan reported 43.4% prevalence of SM with anti-malarials. A Higher prevalence was seen in males, younger age group of <40 years, middle income earners and less educated respondents [39]. Thirty nine percent of the respondents who self-medicated with antimalarials reported incorrect doses and/or inappropriate duration of use of the medication. Self-treatment of malaria is common in Sudan, following self-diagnosis mainly based on presumptive symptoms of malaria. An anti-malarial SM prevalence of 4.4% was reported among university students in Khartoum, Sudan. Higher prevalence was seen in males, among older age group, private university respondents. Twenty eight percent respondents stopped treatment immediately after noticeable improvement, while 71% reported continuing treatment until finishing the medication [51]. A study done among university students in Nigeria reported SM prevalence of 46% with anti-malarials. Higher prevalence self-medication was reported in the older age group, female students, and undergraduate students [93]. High frequency of use of these drugs by the students was expected because they live in a malaria endemic region [93].
Health complaints / clinical indications for which selfmedication is practiced
Cough and cold (61; 43%), body pain (45; 32%), gastrointestinal complaints (44; 31%) and fever (40; 29%) were the top reported health complaints as shown in Table 2.
A nationwide cross-sectional study among teachers from Yemen, Saudi Arabia, and Uzbekistan, reported the SM prevalence ranging from 48% in Saudi Arabia to 78% in Yemen and Uzbekistan. Cough (40%), influenza (33%) and gynecological inflammations (31%) were the top clinical indications for SM with antibiotics. Younger women and those with a lower level of education in Yemen and Saudi Arabia were more likely to keep medicine inventory at home and self-medicate, whereas in Uzbekistan, age and education did not affect self-medication [118]. Pain was the major health complaint among adolescents in public schools in Kuwait. Gender differences were seen in the use of medicines, 64% male students reported SM for muscular pain while 74% of female students reported SM for menstrual discomfort [43]. A study aimed at describing the factors associated with self-medicated consumption of drugs in Spain from a gender perspective revealed 20% prevalence of SM. Cold, influenza and sore throat (31%) were the top clinical indication for SM among women while it was pain (23%) among the men. Women participants with younger age, unmarried status, and having higher education whereas male participants with immigrant status, younger age, less than 7 hours of sleep per day, alcohol consumption, and monthly income of more than 1200 Euros were more likely to SM [76]. A study done among the elderly in Kermanshah-Iran reported 83% prevalence of SM. Pain, cough, and cold were the top clinical indications for SM. Prevalence of SM was in elderly participants who were single, without medical insurance and having higher education. The educated elderly believed that they could get all needed information from medicine brochures or the previous prescriptions; therefore, they diagnosed their sickness and followed self-medication using the same medications [140]. Headache (41%), cold and sore throat (38%), dysmenorrhoea (28%), and fever (25%) were the most frequently reported health conditions for SM use among the high school students in the Czech Republic. Prevalence of SM was more in female students [11]. It was also reported that the mother was the most frequent person who recommended and dispensed the medicines to students [11]. Antibiotic Resistance Surveillance and Control in the Mediterranean (ARMed) project revealed that the overall prevalence of self-medication was 19%, ranging from <0.1% in Cyprus to 37% in Lebanon. The most common indications for SM in nearly all countries were for upper respiratory tract symptoms, fever, ear infection, toothache, diarrhea, urinary tract infections, with the most common antibiotic used being either amoxicillin or ampicillin. The majority of the respondent who self-medicated indicated that they had followed such a practice because they had been given antibiotics for similar symptomatology by a physician [66]. A study of finnish (Finland) people living in Spain revealed antibiotic SM prevalence of 11%, the most common reason for antibiotic use was the common cold (45%), followed by sore throat (17%), bronchitis (10%), urinary tract infection (10%) and dental infection (8%). Antibiotics caused adverse reactions in 17% of the users. The most common self-reported adverse reactions were stomach ache/diarrhoea (5%) and dermatological problems (4%) [46]. Author also pointed out that Fins are not used to buy antibiotics with a prescription in Finland, but in Spain the less regulated pharmacy system makes it possible [46]. Headache (72%), flu (65%), and fever (55%) were the most common symptoms for SM amongst university students from Karachi. Most common SM drug classes for these symptoms were analgesics (88%), antipyretics (65%) and antibiotics (35%). It was also reported that students did not care much about the implications of such behaviour and thus do not hesitate to indulge in SM, which was the reason for no significant differences between SM practices of medical and non-medical students [59].
Sources of SM and recommendation
The most common sources of SM were pharmacy (80; 57%), relatives and friends (45; 32%), left overs (31; 22%), home pharmacy (11; 8%) and previous prescriptions (8; 6%) as shown in Table 2. A study among Chinese university students reported antibiotic SM prevalence of 47%. While most of the students (64.8%) self-medicated based on their own experiences, community pharmacies (89.4%) were the main source for SM. High medical costs at health care centers (generally US$ 32 for receiving care for a common cold, which is nearly 10% of the monthly income per capita for the participants), misconception about antibiotic use for flu-like symptoms, and false confidence in antibiotic use were likely reasons for high SM prevalence [149]. Another study from China to find SM of psoriasis found that television (43.3%), internet (32.7%) and newspapers (22.1%) were the main sources of psoriasis treatment with SM prevalence of 82%. According to this study, doctors spent less than 3 min per patient, which might have prompted patients to seek other ways to obtain information about medications. Discussing the disease with the family significantly reduced the likelihood of SM, probably due to decreased psychosocial burden which built the patient’s confidence to fight psoriasis [130]. A study done in pregnant women from Nigeria reported SM prevalence of 63%. The most frequent sources of the medicines purchased by pregnant women during self-medication were patent medicine stores (55%), pharmacies (30%), and drug hawkers (14%). Seventy-seven percent women regularly kept the medicines at home for the purpose of SM. Mothers-in-law and relatives (41%), patent medicine vendor (20%), pharmacists (12%) and traditional healers (7%) were the frequently cited sources of advice by pregnant women during self-medication. Pregnant women from rural areas where a significant majority of Nigerians reside, have to walk average distance of about 5 km from their residence to the nearest health facility. This was reported as the key factor encouraging self-medication during pregnancy [83]. In contrast to Nigeria, despite the open and rapid access to primary care services, a high prevalence of SM (45%) with antibiotics for treatment of fever and common cold was seen in rural population in Greece. The major source of self-medication was the pharmacy (76%) followed by leftover medications at home (15%) and drugs obtained from relatives or friends (7%) [72]. A study done to find SM for dental problems in Uttar Pradesh, India found SM prevalence of 72%. Major source for SM was pharmacy shop (62%), while other minor sources were mobile drug vendor, hospital pharmacy and patent medical store. Pharmacist (45%), relatives (29%), traditional healers (11%), and personal knowledge (10%) were the main source of motivation for SM [151]. Seventy eight percent prevalence of SM practices was seen among medical and pharmacy students from Jordan. Community pharmacies (75%); leftovers (18%); and friends, relatives (6%) were the main sources of SM. Major sources for instruction on using the medicines were patient leaflet (28%), followed by community pharmacist (20%), students’ study (19%), and previous experience (18%) [152]. A study done to assess the rational use of drugs and self-medication in Turkey found that 59% of patients practiced SM. It was more common among males and more educated patients. 34% of the patients reported that they procured medicines after prescription but without being sick or bought and kept at home in case they were needed. 9% of the patients reported using antibiotics SM, and 56% of them discontinued antibiotic therapy [154]. Eighteen percent people self-medicated antibiotics and 13% used antibiotics for family members in study done in Nigeria. Main sources of SM were pharmacy, and family member or neighbor. Twenty-seven percent people discontinued the antibiotic once the symptoms subsided. Participants with low educational status, younger age, and engagement with a job were more likely to be involved in inappropriate use of antibiotics [133]. A Polish study focused on assessing the knowledge, attitude and perception regarding antibiotics, reported that 10% of patients had antibiotic SM. Major sources of antibiotic SM were leftovers, pharmacy and family and acquaintances [137]. A Lithuanian study revealed almost one-third of the antibiotic users (31%) were involved in SM of antibiotics. SM prevalence was significantly higher in males, and rural participants. It was also noted that the main sources of antibiotic supply were community pharmacies (73%), leftover of antibiotics, and supplies by family members and friends (10%). More than one-tenth of the parents reported administering antibiotics to children according to their own knowledge (8.5%) or advice given by family members and friends (6%) [138]. A study done in Karnataka, India found 30% SM prevalence for treatment of dental conditions. It was reported that the main sources of drugs and instructions for self-medication were the drug vendor (63%), and family and friends (38%) and male respondents were less likely to have undertaken self-medication [139]. A study from Czech Republic reported prevalence of 75% among students of high schools. While the student’s mother was the most frequent person who recommended and dispensed the medicines to students, the home pharmacy cabinet (70%) and pharmacy (16%) were the major sources of SM [11]. Self-Medication prevalence of 69% was reported among parents in Italy, a higher prevalence was seen among females, younger age, and participants with a college degree or higher education. The main source of SM was old prescribed medication (33%) whereas the main source of information on the use of medicines was physician (71%), followed by information leaflet (64%) and pharmacist (40%), internet (16%) and the media (5%) [141]. SM prevalence of 72% was reported from a study among medical students from Nagpur, India. Participants got information about drugs through the reading material (52%) followed by previous prescriptions (17%) and pharmacist (17%). The major sources of drug procurement were pharmacy (75%), free physicians samples (13%), family & friends (11%), and leftovers (8%) [146]. A study from Alexandria, Egypt reported 86% SM prevalence and the major sources of information about drugs were old prescriptions (73%), pharmacist (43%), family or friends (31%) and media, (5%). Drug procurement was from pharmacy, home pharmacy, and leftovers. SM prevalence was higher in participants who were of male sex, with age of 55+ years, and less educated [147]. Sixty one percent prevalence of self-medication was seen among population of Islamabad, Pakistan. Higher prevalence of SM was seen in residents from urban areas, with male sex, those aged between 15-30 years, and literate residents. The most common sources of information on self-medication were own initiative (61%), family/friends (23%), healthcare professionals, (12%), and sales clerk at medical store (4%) [126]. A study to determine the SM among undergraduate students in Thailand reported a SM prevalence of 86%. Television (57%), newspaper (57%), leaflets from hospital (52%) were the top sources for information about drugs for SM. Higher prevalence of SM was seen among girls, adolescents, and in students who lived with parents or relatives [71]. Self-medication prevalence of 26% was reported in a Portuguese urban population. The major sources of recommendations for SM were pharmacist’s advice (50%), own initiative (30%), and friends and relatives (18%). A higher SM prevalence was seen among males, respondents with higher education, students, and employed respondents [8].
Common reasons for SM
Time constraint (59.42%), cost (53; 38%), and mild disease (28.20%) were the top reported reasons for SM as shown in Table 2.
Convenience (86%), cost saving (26%), and lack of trust in prescribing doctor (6%) were the main reasons for SM among Jiangsu University students in China. An antibiotic SM prevalence of 47% was reported in this study. Female gender, older age, and prior knowledge of antibiotics were identified as independent risk factors of SM with antibiotics. Ineffective penalties for contravention, difficulty in surveillance, and poor enforcement of regulations are the main reasons for illegal sale of antibiotics in China [149]. Seventy nine percent prevalence of SM practices was seen among medical and pharmacy students in Jordan. The major reasons for SM were having previous experience with the health problem (56%), believing that the problem is minor (55%), having sufficient medical information (32%), friends advice (17%), and saving time (15%). The majority of these students (83.6%) frequently advised other people about self-medication [152]. A study from Guatemala City did not find a significant difference in antibiotic SM among the higher socioeconomic group (79%) and the lower socioeconomic group (77%). The primary reasons for SM in both groups were time constraints, and purchasing convenience, high cost of visit to doctor, simple disease, and lack of trust towards doctors. Family-friends and pharmacy technicians were the main source for advice of SM for the participants from lower socio-economic status and higher socio-economic status, respectively [134]. Elderly participants from Kermanshah, Iran with a SM prevalence of 83%, cited certainty of its safety (93%), prior experience about the drug (87%), saves time (82%), and non-seriousness of the illness (78%) as the main reasons for SM. Study also pointed out the need for establishment of pharmaceutical consulting unit in pharmacies to inform the elderly about the side effects of self-medication and to change their attitude [140]. The main reasons reported for SM among medical and pharmacy students in Bangladesh were - do not want to burden physician as disease is not important, can manage my disease, want to play an active role regarding own health, advice by relatives, friends, and media, long waiting time at clinic, physician’s last treatment was not successful and lack of trust on physician [18]. Shortages of drugs at health facilities, long waiting time at health facilities, long distance to health facility, inability to pay for health care costs, and freedom to choose the drug of own choice were the main reasons reported to have SM with anti-malarials in rural communities of Kilosa, Tanzania despite the reported decline of malaria [119]. A study among university students from Abbottabad, Pakistan reported, verbal instructions of doctor (45%), previous prescription (39%), no access or time to visit doctor (39%) as the main reasons for SM [108]. University students from the Rio Grande, Brazil reported – having knowledge about disease and drug, simple disease, need for quick relief, physician will prescribe same medication, economy of time and money, unavailability of health service, no trust in health service and opportunity of learning as main reasons for SM [98]. Past experience with similar illness and drugs (53%), convenience (29%), cost (7%) were the main reported reasons for SM with antibiotics in a Jordanian population. Twenty percent participants also stated that they visited second physician to obtain antibiotics when their first physician did not prescribe any [63]. Previous experience (50%), trivial problem (48%), Urgency of problem (31%) were the reported reasons for SM amongst university students of Karachi [59]. First-Year medical students from Arabian Gulf University, Bahrain reported, time-saving (46%), minor illness (25%), and the economical aspect (15%) as the major reasons for SM.
Discussion
In spite of extensive research, number of published reviews and information, along with various rules and regulations in place to control by governmental agencies, self-medication remains a major global problem. SM has multitude of associated problems, some of the major problems are wastage of resources, increased resistance of pathogens and causes for serious health hazards such as adverse reaction and prolonged suffering [156].
Out of total 189279 participants from studies covered in this review, SM was practiced by about 81665 (43%) participants, with the least reported from Sweden (0.3%) [37]. and the highest reported from developing countries such as Bangladesh, Ethiopia, Sri Lanka, and Indonesia. Eighteen percent (25) of the studies did not report SM definition and the remaining studies provided inconsistent phrasing of SM. Further, different study participant groups, varied sample size & age groups, data collection technique differences, low and high response rates have made the SM prevalence review challenging yet interesting.
Thirty-five percent (49) studies did not mention procuring ethical permission for the research. The rights of research participants and the safeguarding of the records of individuals being researched are ethical requirements that must be well documented [157-159]. No research should proceed without consideration of ethical issues anticipated by researchers and without review by an independent and competent ethics review body. These two actions should be considered as integral parts of the research process, and are thus logical and indispensable elements of the study report checklist [160].
Majority of the studies used face to face interview method (98; 70%) for data collection, while self-administered questionnaire technique was used for remaining 42 (30%) studies. Face to face interviews involve more-direct contact, it may be more difficult for the respondent to feel anonymous. This may lead to report more socially desirable answer and underreporting of selfmedication practices [161]. At the same time self-administered questionnaires may fail to get the required information due to limited understanding of the questions, inability of the respondents to get clarifications or details [162]. Authors feel that ethical balance, guaranteed anonymity, proper communication and study explanation to respondents can offer solution to this dilemma.
Before conducing the main study, only 63 (45%) publications reported to have conducted pilot study and there was great variation in the recall periods. This is noteworthy as it is a potential bias for collecting information on self-medication practices. Comparison of recall information about present and past medication use with prospective data from pharmacies and clinics has revealed that respondents had accurate recollections not only about medication use but also start dates of therapy. It was also reported that longer recall period were related to more inaccuracies [163]. However, it is not logical to simply generalize that shorter recall periods are more beneficial. This may also give false negative information as chances of sickness in shorter time frame and hence associated self-medication may be minimal. Longer recall periods may also give additional time points to respondents to give data regarding adverse events, and self-medication diseases. Recall period should be fine-tuned with the help of pilot studies to compare information against available sources such as medical records at pharmacy shops.
Analyzed studies revealed that the most widely self-medicated drugs were antibiotics (83; 59%) and most of the studies (44; 31%) were done to find antibiotic SM prevalence. Although from a public health research viewpoint, this is certainly a positive sign or a way forward by researchers to manage the menace of antibiotic resistance, at the same time it was a disheartening fact that only 9% studies focused on antibiotic resistance awareness among participants. Unlike most of the other drugs that only affect individual patient if used incorrectly for SM, antibiotics add to global risk of increased spread of bacterial resistance [164]. WHO’s global report on antibiotic resistance reveals serious, worldwide threat to public health, it clearly mentions serious threat is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country [165]. While this is true in many cases, responsible self-medication is to be encouraged and can result in positive outcomes as has been reported recently by the WHO and other organizations [165].
Though wealthy countries still use far more antibiotics per capita, high rates in the low- and middle-income countries where surveillance data is now available—such as India, Kenya, and Vietnam—sound a warning to the world. For example, in India, 57 percent of the infections caused by Klebsiella pneumoniae, a dangerous superbug found in hospitals, were found to be resistant to carbepenems, considered as last-resort drug in 2014, up from 29 percent in 2008. For comparison, carbapenems are still effective against Klebsiella infections in 90 percent of cases in the United States and over 95 percent of cases in most of Europe [166]. Even though in developing countries legislation mandates the presentation of a medical prescription for purchase of antibiotics, people can still purchase antibiotics without a prescription [91,112,135,151]. This coupled with low awareness about antibiotic resistance, and associated adverse events contributes to SM with antibiotics in developing countries like but not limited to India, Bangladesh, Pakistan, Ghana, China, Sri Lanka, Nigeria, and Uganda [113,102,118].
In the long term, as health care improves in developing countries, it would then be necessary to improve public awareness and enforce strict antibiotic policies which have yielded positive results in some countries where they were applied [53,168,169]. A good example was in Chile, where to control SM, the Chilean Ministry of Health has strictly restricted the purchase of antibiotics without medical prescription since 1999 [168]. This action resulted in a 43% decrease in antibiotic use in the outpatient setting [168] which represents an impressive result.
High prevalence of antibiotic SM has been seen in southern and eastern European countries that also report high levels of antibiotic resistance. Over the counter sales of antibiotics occur most frequently in Greece, Spain, Malta, Romania (>10% of all sold packages), followed by Bulgaria, Cyprus, Latvia, Lithuania, Poland (1% to 5%), and very rarely in other countries [164]. Major reported source for antibiotic SM among European countries was availability of “leftover” antibiotics [164]. Patient non-compliance and dispensation of larger number of tablets (package based dispensing) rather than actual needed for one single course were the main reasons for leftovers [164].
The World Health Organization recently highlighted the need for country-level antibiotic resistance plans in May 2015 when it endorsed the “Global Action Plan on Antimicrobial Resistance”, which calls on all countries to adopt national strategies within two years [165]. Authors hope that national level action plans to tackle antibiotic resistance along with the findings from available and ongoing research studies will help individual countries to understand the burden of antibiotic resistance in their region and then take coordinated, researchbacked action to limit it.
Reviewed studies showed that considerably higher number of studies enquired about education (82%) while income and health insurance were not reported in 64% and 80% of the studies respectively. Education, income and health insurance have been shown to play an important role in the practices of self-medication [35,64,170]. Studying all three components of socioeconomics i.e., education, income and health insurance will not only help to gain better understanding but also to suggest policy measures in the area of self-medication.
Higher prevalence of SM in educated people reported in many studies is because of their ability to read and understand labels of the consumed medicine, which is almost impossible for the illiterate people [126]. This emphasizes that only education does not improve awareness but there is a need to promote initiatives, such as mass media campaigns and governmental actions, in order to make the citizens comprehend the risks related to the consumption of drugs without medical consultation.
Pagan [170] reported that lack of government-sponsored health insurance coverage increases the propensity to self-medicate. Increasing health insurance coverage could reduce the demand for self-medication by making healthcare more affordable. Unlike study by Pagan, a study from Indonesia reported that participants with health insurance still preferred to SM rather than visit health facilities, even though they had to pay out of pocket [135]. Consultation fees in developing countries are high and high out-of-pocket costs make health services inaccessible to a significant proportion of households. Those accessing health care in the public sector generally receive poor quality services [135]. At the same time much lower density of doctors per 1000 population in, African countries (0.21), Eastern Mediterranean (0.74) and Southeast Asia (0.52) as compared to a density of 3.2 doctors in EU, might contribute to self-medication. In developing country like India, many studies have cited reasons such as high absenteeism, poor quality of services, rampant corruption and long travel distances as prominent reasons for poor access of public sector health facilities [71-73]. This helps to understand, time constraint (59; 42%) and cost (53; 38%), as the top reported reasons for SM reported in the reviewed studies. Therefore, when medications are easily accessible in pharmacies and even in local shops, SM seems a “quick and cheap” method for people’s self-management of their self-diagnosed illness [135].
Conclusion
Authors are of the opinion that this is the first review encompassing the topic of self-medication in the global context, without restricting it to age groups or therapy areas. The review not only gives an insight into the general prevalence of SM but also highlights associated practices and factors indications, reasons, sources, and drugs used. There have been variations in the operational definition of SM, study setting, sample selection, age range, sample size, response rate and recall period. Our review had several limitations that should be recognized. Firstly, we restricted our search only to PubMed Medline and Web of Science databases. Secondly we searched, publications written in only English language. Thirdly, we selected papers published in a time period of year 2000 to 2016.
We wish to point that, high variability seen in the study designs and outcomes emphasize the strong need for multinational cross sectional studies with a standardized methodological approach to gain better comparative understanding of global SM prevalence and practices.
Based on the findings from reviewed studies, we feel that physicians, pharmacists, regulatory authorities, and government should focus more on public health. While implementing the rules and regulations, governments should improve on providing adequate and affordable access to health care services among urban-rural and rich-poor citizens.
Ongoing programs to collect data on medicine consumption and self-medication by consumers, prescription practices of physicians, dispensing drugs without prescription should be designed and implemented. These programs can be integrated into population / disease surveys done in countries. They can also become a routine part in case history collection in day to day physician practice and also in clinical trials. At a pharmacy shop level, prescription monitoring and sales volume comparison should become a routine part of inspection and audit by drug regulatory authorities. As many patients get drug knowledge from previous prescriptions, physicians should limit unnecessary prescriptions of antibiotics, other drugs and implement good prescribing practices.[172] By using the available technology and user friendliness of cell phones and cell phone apps, apps could be designed to send prescriptions from physician to pharmacist based on which dispensing would occur to consumers. This method would limit the reuse of prescription and also will help to store the data, which could be monitored and audited by drug regulatory authorities.
Authors would also like to mention that, not only developing and conducting the programs but getting the feedback, understanding the lacunas, continuous improvisation, measuring the improvement in knowledge of people and their self-medication practices is needed for control of the menace of self-medication.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Agbor M, Azodo C. Self-medication for oral health problems in Cameroon. Int Dent J. 2011; 61 :204-209.
- World Health Organization. Guidelines for the regulatory assessment of Medicinal Products for use in self-medication 2000.
- Afridi M, Rasool G, Rabia Tabassum R, et al. Prevalence and pattern of self-medication in Karachi: A community survey. Pak J Med Sci. 2015; 31: 1241-1245.
- Loyola Filho AI, Lima-Costa MF. Uchoˆa Bambuı´ Project: A qualitative approach to self-medication. Cad Saude Publica 2004;20: 1661-1669.
- Gualano M, Bert F, Passi S, et al. Use of self-medication among adolescents: a systematic review and meta-analysis. European Journal of Public Health, 2014; 25 444-450.
- Sharma A, Oommen S, Topno I, et al. Perceptions and practices of self-medication in healthcare and nonhealthcare university students in South India. J Basic Clin Physiol Pharmacol. 2015; 26:633-640.
- Sahebi L, Vahidi R. Self-medication and storage of drugs at home among the clients of drugstores in Tabriz. Current Drug Safety. 2009; 4:107-112.
- Martins A, Miranda A, Mendes Z, et al. Self-medication in a Portuguese urban population: A prevalence study. Pharmaco-epidemiology and drug safety 2002; 11: 409-414.
- Grigoryan L, Burgerhof J, Haaijer-Ruskamp F, et al. Is self-medication with antibiotics in Europe driven by prescribed use? Journal of Antimicrobial Chemotherapy 2007; 59, 152-156.
- Grigoryan L, Haaijer-Ruskamp F Burgerhof J. Self-medication with antimicrobial drugs in Europe. Emerging Infectious Diseases. 2006; 12:452-459.
- Kopecna E, Mica M, Vlcek J. Use of medicines among students of high schools in the Czech Republic. Acta Pol Pharm. 2015;72:389-96.
- Kumar N, Kanchan T, Unnikrishnan B. Perceptions and practices of self-medication among medical students in coastal South India. PLoS ONE 8: e72247.
- Al-Rasheed A, Umar Yagoub U, Al-Khashan H, et al. Prevalence and predictors of self-medication with antibiotics in Al-Wazarat Health Center, Riyadh City, KSA. Biomed Res Int. 2016; 2016:3916874. doi: 10.1155/2016/3916874. Epub 2016 Jan 5.
- Oshikoya K, Senbanjo I, Njokanma O. Self-medication for infants with colic in Lagos, Nigeria. BMC Pediatrics 2009, 9:9.
- James H, Handu S, Khalid A. et al. Evaluation of the knowledge, attitude and practice of self‐medication among first-year medical students. Med Princ Pract. 2006; 15:270-275.
- Awad A, Eltayeb I. Self-medication practices in Khartoum state, Sudan. Eur J Clin Pharmacol. 2006; 62:317-324.
- Suleman S, Ketsela A, Mekonnen Z. Assessment of self-medication practices in Assendabo town, Jimma zone, southwestern Ethiopia. Research in Social and Administrative Pharmacy. 2009; 5:76-81.
- Alam N, Saffoon N, Uddin R. Self-medication among medical and pharmacy students in Bangladesh. BMC Res Notes (2015) 8:763.
- Shehnaz S, Agarwal A, Khan N. A systematic review of self-medication practices among adolescent. J Adolesc Health. 2014 Oct;55:467-483.
- Jerez-Roig J, Medeiros L, Silva V, et al. Prevalence of self-medication and associated factors in an elderly population: a systematic review. Drugs Aging. 2014 Dec;31:883-896.
- Montgomery A, Bradley C, Rochfort A, et al. A review of self-medication in physicians and medical students. . Occup Med (Lond). 2011 Oct;61:490-497.
- Azami-Aghdash S, Mohseni M, Etemadi M, et al. Prevalence and cause of self-medication in Iran: A systematic review and meta-analysis article. Iran J Public Health. 2015 Dec; 44:1580-1593.
- Domingues P, Galvão T, Andrade K, et al. Prevalence of self-medication in the adult population of Brazil: a systematic review. Rev Saude Publica. 2015; 49:36.
- Hinz B. Self-medication of headaches. Med Monatsschr Pharm. 2015; 38:439-441.
- Ocan M, Obuku E, Bwanga F, et al. Household antimicrobial self-medication: A systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health. 2015; 1;15:742.
- Corrêa-Fissmer M, Mendonça M, Martins A, et al. Prevalence of self-medication for skin diseases: a systematic review. An Bras Dermatol. 2014 Jul-Aug;89:625-630.
- Jain S, Malvi R, Purviya J. Concept of self-medication: A review. Int. J. Pharm Biol Arch. 2011; 2: 831-836.
- Sanchez J. Self-medication practices among a sample of Latino migrant workers in South Florida. Front Public Health. 2014; 2:108. doi: 10.3389/fpubh.2014.00108. eCollection 2014.
- Hassali MA, Shafie AA, Al-Qazaz H. Et al. Self-medication practices among adult population attending community pharmacies in Malaysia: An exploratory study. Int J Clin Pharm. 2011; 33:794-799.
- Centre for Reviews and Dissemination: Systematic reviews: CRD’s guidance for undertaking reviews in health care: Centre for Review and Dissemination. York: University of York; 2009.
- Moher D, Liberati A, Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J Clin Epidemiol.2009; 62:1006-1012.
- Bi P, Tong S, Parton K. Family self-medication and antibiotics abuse for children and juveniles in a Chinese city. Social Science and Medicine. 2000; 50:1445-1450.
- Hayran O, Karavus M, Aksayan S. Help-Seeking Behavior and Self-Medication of a Population in an Urban Area in Turkey: A cross sectional study. Croat Med J 2000; 41:327-332.
- Figueiras A, Caamano F, Gestal-Otero J. Socio-demographic factors related to self-medication in Spain. European Journal of Epidemiology. 2000; 16: 19-26.
- Shankar P, Partha P, Shenoy N. Self-medication and non-doctor prescription practices in Pokhara valley, Western Nepal: a questionnaire-based study. BMC Fam Pract. 2002;17;3:17.
- Buke A, Ermertcan S, Hosgor-Limoncu M. Rational antibiotic use and academic staff. International Journal of Antimicrobial Agents. 2003;21:63-66.
- Svensson E, Haaijer-Ruskamp F, Lundborg C. Self-medication with antibiotics in a Swedish general population. Scand J Infect Dis. 2004;36(6-7):450-452.
- Raz R, Edelstein H, Grigoryan L, Haaijer-Ruskamp F. Self-medication with antibiotics by a population in northern Israel. Isr Med Assoc J. 2005;7:722-725.
- Awad A, Eltayeb I, Matowe L, Thalib L. Self-medication with antibiotics and anti-malarials in the community of Khartoum State, Sudan. J Pharm Pharm Sci. 2005;12;8:326-331.
- Akanbi O, Odaibob A, Afolabi K. Effect of self-medication with antimalarial drugs on malaria infection in pregnant women in South-Western Nigeria. Med Princ Pract 2005;14:6-9
- Aljinoviæ-Vuèiæ V, Trkulja V, Lackoviæ Z. Content of home pharmacies and self-medication practices in households of pharmacy and medical students in Zagreb, Croatia: Findings in 2001 with a Reference to 1977. Croat Med J 2005;46:74-80.
- Linden K, Jormanainen V, Swigonski N, Pietilä K. Self-medication among Finnish young men in the beginning of common military service. Pharmacoepidemiol Drug Saf. 2005;14:193-201.
- Abahussain E, Matowe L, Nicholls P. Self-reported medication use among adolescents in Kuwait. Med Princ Pract 2005;14:161-164.
- Muscat M, Monnet D, Klemmensen T, et al. Patterns of antibiotic use in the community in Denmark. Scandinavian Journal of Infectious Diseases. 2006; 38: 597-603.
- Sihavong A, Lundborg C, Syhakhang L, et al. Antimicrobial self-medication for reproductive tract infections in two provinces in Lao People’s Democratic Republic. Sex Transm Infect 2006;82:182-186.
- Väänänen M, Pietilä K, Airaksinen M. Self-medication with antibiotics--does it really happen in Europe? Health Policy. 2006;77:166-171.
- Sedighi B, Ghaderi-Sohi S, Sara Emami S. Evaluation of self-medication prevalence, diagnosis and prescription in migraine in Kerman, Iran. Neurosciences 2006; Vol. 11 : 84-87.
- de Melo M, Madureira B, Ferreira A. et al. Prevalence of self-medication in rural areas of Portugal. Pharm World Sci. 2006;28:19-25.
- Grigoryan L, Burgerhof J, Degener J, et al. Attitudes, beliefs and knowledge concerning antibiotic use and self-medication: a comparative European study. Pharmacoepidemiology and drug safety 2007; 16: 1234-1243.
- Esimone C, Nworu C, Udeogaranya O. Utilization of antimicrobial agents with and without prescription by out-patients in selected pharmacies in South-eastern Nigeria. Pharm World Sci (2007) 29:655-660.
- Awad A, Eltayeb I. Self-medication practices with antibiotics and antimalarials among Sudanese undergraduate university students. Ann Pharmacother. 2007;41:1249-1255.
- Pereira F, Bucaretchi F, Stephan C, et al. Self-medication in children and adolescents. J Pediatr (Rio J). 2007;83:453-458.
- Kitikannakorn N, Chanthonrat C. Knowledge and accessibility of self-medication among undergraduate students in Thailand. International Journal on Disability and Human Development.2007; 6:289-294.
- Lal V, Goswami A, Anand K. Self-medication among residents of urban resettlement colony, New Delhi. Indian J Pub Health. 2007; 51:249-251.
- Grigoryan L, Burgerhof J, Degener J. Determinants of self-medication with antibiotics in Europe: the impact of beliefs, country wealth and the healthcare system. Journal of Antimicrobial Chemotherapy. 2008; 61: 1172-1179.
- Awad A, Al-Rabiy S, Abahussain E. Self-medication practices among diabetic patients in Kuwait. Med Princ Pract. 2008;17:315-320.
- James H, Handu S, Khaja K, Sequeira R. Influence of medical training on self-medication by students. Int J ClinPharmacolTher. 2008;46:23-29.
- Tourinho F, Bucaretchi F, Stephan C. Home medicine chests and their relationship with self-medication in children and adolescents. J Pediatr (Rio J). 2008;84:416-422.
- Zafar S, Syed R, Waqar S. et al. Self-medication amongst university students of Karachi: prevalence, knowledge and attitudes. J Pak Med Assoc. 2008;58:214-217.
- Yousef A, Al-Bakri A, Bustanji Y, Wazaify M. Self-medication patterns in Amman, Jordan. Pharm World Sci. 2008;30:24-30.
- Sawalha A. A descriptive study of self-medication practices among Palestinian medical and nonmedical university students. Res Social Adm Pharm. 2008;4:164-172.
- Coffman M, Shobe M, O’Connell B. Self-prescription practices in recent Latino immigrants. Public Health Nursing. 2008; 25:203-211.
- Sawair F, Baqain Z, Karaky A. Assessment of self-medication of antibiotics in a Jordanian Population. Med Princ Pract 2009;18:21-25.
- Ilhan M, Durukan E, Ilhan S. Self-medication with antibiotics: questionnaire survey among primary care center attendants. Pharmacoepidemiol Drug Saf. 2009;18:1150-1157.
- Mainous A, Diaz V, Carnemolla M. A community intervention to decrease antibiotics used for self-medication among Latino adults. Ann Fam Med. 2009; 7: 520-526.
- Scicluna E, Borg M, Gür D. et al. Self-medication with antibiotics in the ambulatory care setting within the Euro-Mediterranean region; results from the AR Med project. J Infect Public Health. 2009;2:189-197.
- Abasaeed A, Vlcek J, Abuelkhair M, Kubena A. Self-medication with antibiotics by the community of Abu Dhabi Emirate, United Arab Emirates. J Infect Dev Ctries. 2009;3:491-497.
- Chaves R, Lamounier J, César C. Self-medication in nursing mothers and its influence on the duration of breastfeeding. J Pediatr (Rio J). 2009;85:129-134.
- Barros A, Griep R, Rotenberg L. Self-medication among nursing workers from public hospitals. Rev Latino-am Enfermagem. 2009; 17:1015-1022.
- Balbuena F, Aranda A, Figueras A. Self-medication in older urban mexicans : an observational, descriptive, cross-sectional study. Drugs Aging. 2009;26:51-60.
- Kitikannakora N, Sitthiworranan C. Self-medication among undergraduate students in Thailand. Int J Disabil Hum Dev 2009;8:411-416.
- Skliros E, Merkouris P, Papazafiropoulou A. Self-medication with antibiotics in rural population in Greece: a cross-sectional multicenter study. BMC Fam Pract. 2010;11:58.
- Sarahroodi S, Arzi A, Sawalha A. Antibiotics self-medication among southern Iranian University Students. International Journal of Pharmacology. 2010; 6:48-52.
- Landers T, Ferng Y, McLoughlin J. Antibiotic identification, use, and self-medication for respiratory illnesses among urban Latinos. Journal of the American Academy of Nurse Practitioners.2010; 22: 488-495.
- Sapkota A, Coker M, Goldstein R. Self-medication with antibiotics for the treatment of menstrual symptoms in southwest Nigeria: A cross-sectional study. BMC Public Health 2010, 10:610.
- Carrasco-Garrido P, Herna´ndez-Barrera V, De Andre´s A, et al. Sex—Differences on self-medication in Spain. pharmacoepidemiology and drug safety. 2010; 19: 1293-1299.
- Klemenc-Ketis Z, Hladnik Z, Kersnik J. Self-medication among healthcare and non-healthcare students at University of Ljubljana, Slovenia. Med Princ Pract. 2010;19:395-401.
- Klemenc-Ketis Z, Kersnik J. Sources and predictors of home-kept prescription drugs. International Journal of Clinical Pharmacology and Therapeutics. 2010; 48:705-707.
- Smogavec M, Softič N, Kersnik J, Klemenc-Ketiš Z. An overview of self-treatment and self-medication practices among Slovenian citizens. Zdrav Vestn 2010; 79: 757-763.
- Schmid B, Bernal R, Silva N. Self-medication in low-income adults in Southeastern Brazil. Rev Saúde Pública 2010;44: 1039-1045.
- Adedapo H, Lawal A, Adisa A, Adeyemi B. Non-doctor consultations and self-medication practices in patients seen at a tertiary dental center in Ibadan. Indian J Dent Res. 2011;22:795-798.
- Afolabi O, Ehalaiye B, Fadare J. Survey of ototopical self-medication among patients attending ENT and family medicine departments in a Nigerian hospital. Eur J Gen Pract. 2011;17:167-170.
- Yusuff K, Omarusehe L. Determinants of self-medication practices among pregnant women in Ibadan, Nigeria. Int J Clin Pharm. 2011;33:868-875.
- Hussain S, Malik F, Ashfaq K, et al. Prevalence of self-medication and health-seeking behavior in a developing country. African Journal of Pharmacy and Pharmacology. 2011;5:972-978.
- de Moraes A, Delaporte T, Molena-Fernandes C, et al. Factors associated with medicine use and self medication are different in adolescents. Clinics 2011;66:1149-1155.
- Wen Y, Lieber E, Wan D. A qualitative study about self-medication in the community among market vendors in Fuzhou, China. Health and Social Care in the Community. 2011; 19: 504-513.
- Bang S, Sontakke S, Thawani V. Pre and post-interventional pattern of self-medication in three common illnesses in staff of a tertiary hospital. Indian Journal of Pharmacology. 2011; 43:275-277.
- Klemenc-Ketis Z, Hladnik Z, Kersnik J. A cross sectional study of sex differences in self-medication practices among university students in Slovenia. CollAntropol. 2011; 35 2: 329-334.
- Souza L, Da Silva C, Ferraz G, et al. The prevalence and characterization of self-medication for obtaining pain relief among undergraduate nursing students. Rev Latino-Am Enfermagem. 2011; 19:245-51.
- Pan H, Cui B, Zhang D. et al. Prior knowledge, older age, and higher allowance are risk factors for self-medication with antibiotics among university students in southern China. PLoS One. 2012;7:e41314.
- Donkor E, Tetteh-Quarcoo P, Nartey P, Agyeman I. Self-medication practices with antibiotics among tertiary level students in Accra, Ghana: a cross-sectional study. Int J Environ Res Public Health. 2012; 9:3519-3529.
- Grosso G, Marventano S, Ferranti R, et al. Pattern of antibiotic use in the community: Νon-adherence and self-prescription rates in an Italian urban population. Molecular Medicine Reports. 2012; 5:1305-1310.
- Osemene K, Lamikanra A. A study of the prevalence of self-medication practice among university students in Southwestern Nigeria. Trop J Pharm Res, August 2012; 11: 683- 689.
- Mehuys E, Paemeleire K, Van Hees T. et al. Self-medication of regular headache: a community pharmacy - based survey. Eur J Neurol. 2012;19:1093-1099.
- González-López J, Rodríguez-Gázquez M, Lomas-Campos M. Self-medication in adult Latin American immigrants in Seville. Acta Paul Enferm. 2012; 25:75-81.
- Bano N, Najan R, Qazi F. Irrational drug use based on self-medication for some common clinical conditions in an educated population of Karachi. Pak J Med Sci. 2012;28:359-362.
- Horton S, Stewart A. Reasons for self-medication and perceptions of risk among Mexican migrant farm workers. J Immigrant Minority Health (2012) 14:664-672.
- Da Silva M, Soares M, Muccillo-Baisch A. Self-medication in university students from the city of Rio Grande, Brazil. BMC Public Health. 2012;8:339.
- Banerjee I, Bhadury T. Self-medication practice among undergraduate medical students in a tertiary care medical college, West Bengal. J Postgrad Med. 2012;58:127-131.
- Marquez G, Torres V, Sanchez V. et al. Self-medication in Ophthalmology: A Questionnaire-based Study in an Argentinean Population. Ophthalmic Epidemiology. 2012; 19, 236-241.
- Kivelevitch D, Tahhan P, Bourren P, et al. Self-medication and adherence to treatment in psoriasis. Int J Dermatol. 2012;51:416-419.
- Muras M, Krajewski J, Nocun M, Godycki-Cwirko M. A survey of patient behaviours and beliefs regarding antibiotic self-medication for respiratory tract infections in Poland. Arch Med Sci. 2013;31;9:854-857.
- Napolitano F, Izzo M, Di Giuseppe G, et al. Public knowledge, attitudes, and experience regarding the use of antibiotics in Italy. PLOS ONE. 2013; 8:e84177.
- Al-Ramahi R. Patterns and attitudes of self-medication practices and possible role of community pharmacists in Palestine. Int J Clin Pharmacol Ther. 2013;51:562-567.
- Araújo D, Leal M, Santos E. Consumption of medicines in high-risk pregnancy: evaluation of determinants related to the use of prescription drugs and self-medication. Brazilian Journal of Pharmaceutical Sciences. 2013; 49:491-499.
- Baghianimoghadam M, Mojahed S, Baghianimoghadam M. Attitude and practice of pregnant women regarding self-medication in Yazd, Iran. Arch Iran Med. 2013;16:580-583.
- Asseray N, Ballereau F, Trombert-Paviot B. Frequency and severity of adverse drug reactions due to self-medication: a cross-sectional multicentre survey in emergency departments. Drug Saf. 2013;36:1159-1168.
- Ulah H, Khan S, Ali S. Evaluation of self-medication amongst university students in Abbottabad, Pakistan; prevalence, attitude and causes. Acta Poloniae Pharmaceutica ñ Drug Research. 2013; 70: 919-922.
- Santos T, Lima D, Nakatani A, et al. Medicine use by the elderly in Goiania, Midwestern Brazil. Rev Saúde Pública 2013; 47: 94-103.
- Alexa I, Pancu A, Moroşanu A. et al. The impact of self-medication with NSAIDS / analgesics in a north-eastern region of romania. Farmacia, 2014;62:1164-1170.
- Brlić K, Janev N, Sović S, Štimac D. Characteristics of self-medication for pain relief among first - year health care students in Zagreb, Croatia. Psychiatr Danub. 2014;26:459-465.
- Lv B, Zhou Z, Xu G, et al. Knowledge, attitudes and practices concerning self-medication with antibiotics among university students in western China. Trop Med Int Health. 2014; 19:769-779.
- Shah S, Ahmad H, Rehan R. et al. Self-medication with antibiotics among non-medical university students of Karachi: a cross-sectional study. BMC Pharmacol Toxicol. 2014;23;15:74.
- Cheaito L, Azizi S, Saleh N, Salameh P. Assessment of self-medication in population buying antibiotics in pharmacies: a pilot study from Beirut and its suburbs. Int J Public Health. 2014;59:319-327.
- Ocan M, Bwanga F, Bbosa G. et al. Patterns and predictors of self-medication in northern Uganda. PLoS One. 2014;9:e92323.
- Biswas M, Roy M, Manik M, et al. Self-medicated antibiotics in Bangladesh: a cross-sectional health survey conducted in the Rajshahi City. BMC Public Health. 2014 Aug 14;14:847. doi: 10.1186/1471-2458-14-847.
- Ramalhinho I, Cordeiro C, Cavaco A, Cabrita J. Assessing determinants of self-medication with antibiotics among Portuguese people in the Algarve Region. Int J Clin Pharm. 2014;36:1039-1047.
- Belkina T, Al Warafi A, Hussein E, et al. Antibiotic use and knowledge in the community of Yemen, Saudi Arabia, and Uzbekistan. J Infect Dev Ctries. 2014 Apr 15;8:424-9. doi: 10.3855/jidc.3866.
- Chipwaza B, Mugasa J, Mayumana I, et al. Self-medication with anti-malarials is a common practice in rural communities of Kilosa district in Tanzania despite the reported decline of malaria. Malaria Journal. 2014; 13:252.
- Martinez J, Pereira G, Ribeiro L. et al. Study of self-medication for musculoskeletal pain among nursing and medicine students at Pontifícia Universidade Católica - São Paulo. Rev Bras Reumatol. 2014;54:90-94.
- Eticha T, Mesfin K. Self-medication practices in Mekelle, Ethiopia. PLoS One. 2014;9:e97464.
- Khan H, Maheen S, Alamgeer. Determinants of Increasing Trend of Self-Medication in a Pakistani Community. Trop J Pharm Res 2014; 13: 437-443.
- Bertoldi A, Camargo A, Silveira M. et al. Self-medication among adolescents aged 18 years: the 1993 Pelotas (Brazil) birth cohort study. J Adolesc Health. 2014; 55:175-181.
- Lukovic J, Miletic V, Pekmezovic T. Self-medication practices and risk factors for self-medication among medical students in Belgrade, Serbia. PLoS One. 2014;11;9:e114644.
- Machado-Alba J, Echeverri-Cataño L, Londoño-Builes M, et al. Social, cultural and economic factors associated with self-medication. Biomédica 2014;34: 580-588.
- Aqeel T, Shabbir A, Basharat H. Prevalence of Self-Medication among Urban and Rural Population of Islamabad, Pakistan. Trop J Pharm Res. 2014; 13: 627-633.
- Chenge M, der Vennet J, Luboya N, et al. Health-seeking behaviour in the city of Lubumbashi, Democratic Republic of the Congo: results from a cross-sectional household survey. BMC Health Services Research201414:173.
- Papakosta M, Zavras D, Niakas D. Investigating factors of self-care orientation and self-medication use in a Greek rural area. Rural Remote Health. 2014;14:2349.
- Foroutan B, Foroutan R. Household storage of medicines and self-medication practices in south-east Islamic Republic of Iran. East Mediterr Health J. 2014;20:547-553.
- Zhang L, Yang H, Wang Y. Self-medication of psoriasis in southwestern China. Dermatology. 2014; 228:368-374.
- Ibrahim N, Alamoudi B, Baamer WO, Al-Raddadi R. Self-medication with analgesics among medical students and interns in King Abdulaziz University, Jeddah, Saudi Arabia. Pak J Med Sci. 2015;31:14-18.
- Gunawardhana C, Sakeena M, Sivayoganthan C. Awareness of rational medication use and antibiotic self-medication practices among undergraduate students in a university in Sri Lanka. Trop J Pharm Res, 2015; 14: 723-729.
- Gebeyehu E, Bantie L, Azage M. Inappropriate Use of Antibiotics and Its Associated Factors among Urban and Rural Communities of Bahir Dar City Administration, Northwest Ethiopia. PLoS One. 2015; 17;10:e0138179.
- Ramay B, Lambour P, Cerón A. Comparing antibiotic self-medication in two socio-economic groups in Guatemala City: a descriptive cross-sectional study. BMC Pharmacol Toxicol. 2015;16:11.
- Widayati A, Suryawati S, de Crespigny C, Hiller J. Self-medication with antibiotics in Yogyakarta City Indonesia: a cross sectional population-based survey. BMC Res Notes. 2011;4:491.
- Awad A, Aboud E. Knowledge, Attitude and Practice towards Antibiotic Use among the Public in Kuwait. PLoS One. 2015; 10: e0117910.
- Drozd M, Drozd K, Filip R. Knowledge, attitude and perception regarding antibiotics among polish patients. Acta Pol Pharm. 2015; 72:807-817.
- Pavydė E, Veikutis V, Mačiulienė A, et al. Public Knowledge, Beliefs and Behavior on Antibiotic Use and Self-Medication in Lithuania. Int J Environ Res Public Health. 2015; 17;12:7002-7016.
- Simon A, Rao A, Rajesh G, et al. Trends in self-medication for dental conditions among patients attending oral health outreach programs in coastal Karnataka, India. Indian J Pharmacol. 2015; 47:524-529.
- Jafari F, Khatony A, Rahmani E. Prevalence of self-medication among the elderly in Kermanshah-Iran. Glob J Health Sci. 2015 Jan 21;7:360-5.
- Garofalo L, Di Giuseppe G, Angelillo I. Self-Medication Practices among Parents in Italy. BioMed Research International. Volume 2015 (2015), Article ID 580650, 8 pages.
- Aljadhey H, Assiri G, Mahmoud M. Self-medication in central Saudi Arabia community pharmacy consumers’ perspectives. Saudi Med J. 2015; 36: 328-334.
- Kusturica M, Tomic Z, Bukumiric Z, et al. Home pharmacies in Serbia: an insight into self-medication practice. Int J Clin Pharm. 2015; 37:373-378.
- ALBashtawy M, Batiha A, Tawalbeh L, et al. Self-medication among school students. J Sch Nurs. 2015 Apr;31:110-116.
- Nguyen H, Nguyen T. Factors associated with self-medication among medicine sellers in urban Vietnam. Int J Health Plann Manage. 2015; 30:219-231.
- Kasulkar A, Gupta M. Self-medication practices among medical students of a private institute. Indian J Pharm Sci. 2015; 77: 178-182.
- El-Nimr N, Wahdan I, Wahdan A. Self-medication with drugs and complementary and alternative medicines in Alexandria, Egypt: prevalence, patterns and determinants. East Mediterr Health J. 2015; 21:256-65.
- Abeje G, Admasie C, Wasie B. Factors associated with self-medication practice among pregnant mothers attending antenatal care at governmental health centers in Bahir Dar city administration, Northwest Ethiopia, a cross sectional study. Pan Afr Med J. 2015;20:276. doi: 10.11604/pamj.2015.20.276.4243.
- Zhu X, Pan H, Yang Z. Self-medication practices with antibiotics among Chinese university students. Public Health. 2016; 130:78-83.
- Ali A, Ahmed J, Ali A. Practices of self-medication with antibiotics among nursing students of Institute of Nursing, Dow University of Health Sciences, Karachi, Pakistan. J Pak Med Assoc. 2016; 66:235-237.
- Jain A, Bhaskar D, Gupta D, et al. Practice of Self-Medication for Dental Problems in Uttar Pradesh, India. Oral Health Prev Dent. 2016; 14:5-11.
- Alkhatatbeh M, Alefan Q, Alqudah M. High prevalence of self-medication practices among medical and pharmacy students: a study from Jordan. Int J Clin Pharmacol Ther. 2016; 54:390-398.
- Panda A, Pradhan S, Mohapatra G. Drug-related problems associated with self-medication and medication guided by prescription: A pharmacy-based survey. Indian J Pharmacol. 2016; 48: 515-521.
- Nayir T, Okyay R, Yesilyurt H. Assessment of rational use of drugs and self-medication in Turkey: A pilot study from Elazıg and its suburbs. Pak J Pharm Sci. 2016; 29:1429-1435.
- Filipe V, Allen P, Peyrin-Biroulet L. Self-medication with steroids in inflammatory bowel disease. Dig Liver Dis. 2016;48:23-26.
- Bennadi D. Self-medication: A current challenge. J Basic Clin Pharma 2014;5:19-23.
- The World Medical Association. Declaration of Helsinki. Ethical principles for medical research involving human subjects. General Assembly 2008. Ferney-Voltaire, France: WMA, 2012. http://www.wma.net/ en/30publications/10policies/b3/index.html (Accessed February 2012).
- Council for International Organisations of Medical Sciences. International ethical guidelines for biomedical research involving human subjects. Geneva, Switzerland: CIOMS, 2002. http://www.cioms.ch/publications/ guidelines/guidelines_nov_2002_blurb.htm (Last accessed on February 2012).
- US Department of Health and Human Services, Office for Human Research Protections. Code of federal regulations. Title 45: public welfare. Part 46: protection of human subjects. Washington DC, USA: HHS, OHRP, Rev January 2009. www.hhs.gov/ohrp/policy/index.html Accessed February 2012.
- Edginton M, Enarson D, Zachariah R. Why ethics is indispensable for good-quality operational research. PHA 2012; 2: 21-22.
- Bowling A. Mode of questionnaire administration can have serious effects on data quality. Journal of Public Health. 2005; 27:281-291.
- Nieuwenhuijsen M. Design of exposure questionnaires for epidemiological studies. Occup Environ Med 2005;62:272-280.
- Strom B, Kimmel S, Hennessy S. Special issues in pharmacoepidemiology methodology in textbook of pharmacoepidemiology. John Wiley & Sons. 2ndedt. 2013:214.
- Grigoryan L, Monnet D, Haaijer-Ruskamp F. Self-medication with antibiotics in Europe: A case for action. Curr Drug Saf. 2010 Oct;5:329-32.
- Antimicrobial Resistance Global Report on Surveillance. WHO. 2014
- White A. CDDEP Maps Dangerous Trends in Antibiotic Resistance on a Global Scale for the First Time. 2015
- Kafle K, Gartoulla R: Self-medication and its impact on essential drugs schemes in Nepal: A Socio-Cultural Research Project: Action Programme on Essential Drugs. Geneva: World Health Organization; 1993.
- Bavestrello L, Cabello A, Casanova D. Impact of regulatory measures in the trends of community consumption of antibiotics in Chile. Rev Med Chil. 2002, 130, 1265-1272.
- Kardas P, Devine S, Golembesky A. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. Int J Antimicrob Agents. 2005; 26:106-113.
- Pagán J, Ross S, Yau J. Self-medication and health insurance coverage in Mexico. Health Policy. 2006;75:170-177.
- Peters D, Garg A, Bloom G. Poverty and access to health care in developing countries. Ann NY Acad Sci. 2008;1136: 161-171.
- De Vries T, Henning R, Hogerzeil H, et al. Guide to good prescribing. A practical manual. World Health Organization. Action Programme on Essential Drugs. Geneva. 1994.

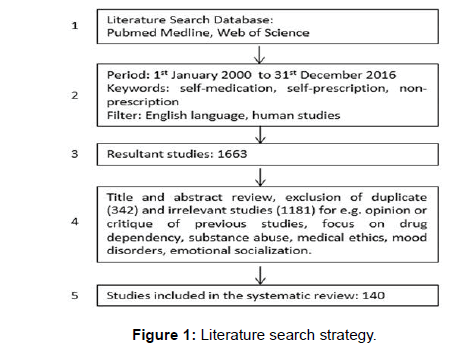



 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.