Adnexal Tumors of Skin: An Experience at a Tertiary Care Center at Delhi
2 Department of Pathology, Dr. RML Hospital, New Delhi, India, Email: madani.mahla@yahoo.com
3 Department of Pathology, Hamdard Institute of Medical Sciences and Research, New Delhi, India, Email: jairajpuri@jamiahamdard.ac.in
Citation: Pujani M, Madaan GB, Jairajpuri ZS, Jetley S, Hassan MJ, Khan S. Adnexal tumors of skin: An experience at a tertiary care center at Delhi. Ann Med Health Sci Res 2016;6:280-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Adnexal skin tumors are a heterogeneous group of uncommon tumors usually misdiagnosed clinically due to a huge variety of types and their variants. Histopathology usually helps in establishing the diagnosis. Aims: The study was undertaken to analyze the morphological, clinical, and histological features of adnexal tumors (ATs) of the skin at our center over a period of 4 years. Subjects and Methods: retrospective study was conducted over a period of 4 years (April 2010–March 2014), comprising 25 ATs of skin diagnosed in the Department of Pathology, Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, New Delhi. All the consecutively reported AT cases were reviewed and reclassified as AT arising from sebaceous glands, hair follicles, or sweat glands. The concordance of clinical and histopathological diagnosis was also assessed. Results: Most of the ATs were benign (24/25) with head and neck being the most common location (72%). Nearly 56% of the tumors exhibited sweat gland differentiation, 28% hair follicle differentiation, and sebaceous gland tumors accounted for 16%. The most common varieties of tumors encountered in the present study included hidradenoma papilliferum and pilomatricoma. The concordance between clinical and histopathological diagnosis was found to be 50% approximately. Conclusions: ATs of the skin are rare neoplasms with benign tumors being far more common. They are often misdiagnosed clinically, so histopathology remains the gold standard for establishing an accurate diagnosis of skin ATs.
Keywords
Adnexal tumors, hair follicle differentiation, sebaceous, sweat gland
Introduction
Adnexal tumors (ATs) are the tumors arising from the appendages of the skin such as sweat glands, sebaceous glands, and hair follicles. Adnexal skin tumors are usually misdiagnosed clinically, and histopathology usually provides diagnostic confirmation. ATs are a heterogeneous group of skin tumors with mostly benign behavior. These are usually found as solitary, sporadic lesions; however, certain specific types of multiple tumors maybe an indication of some complex genetic syndromes, for example, Cowden’s syndrome and Muir Torre syndrome.[1,2] Occasionally, a significant association has been observed with certain internal malignancies.[3]
This study was undertaken to analyze the morphological, clinical, and histological features of ATs of the skin at our center over a period of 4 years.
Subjects and Methods
The present study was a retrospective study over a period of 4 years (April 2010–March 2014), including all the ATs of the skin diagnosed in the Department of Pathology at Hamdard Institute of Medical Sciences and Research, Jamia Hamdard, New Delhi. All the consecutively histopathologically diagnosed ATs of the skin, irrespective of their clinical diagnoses, were included in the study.
Patients’ clinical details such as age, sex, provisional clinical diagnosis, size, and location were documented. The excised specimens were subjected to gross and microscopic examination using hematoxylin and eosin stain. A few cases were subjected to periodic acid-Schiff (PAS) stain and alcian blue stain as and when required for establishing a correct diagnosis. All the slides were reviewed and reclassified as AT arising from sebaceous glands, hair follicles, or sweat glands. The concordance of clinical and histopathological diagnosis was also assessed. If clinical suspicion of AT was kept before biopsy, such cases were considered concordant.
Results
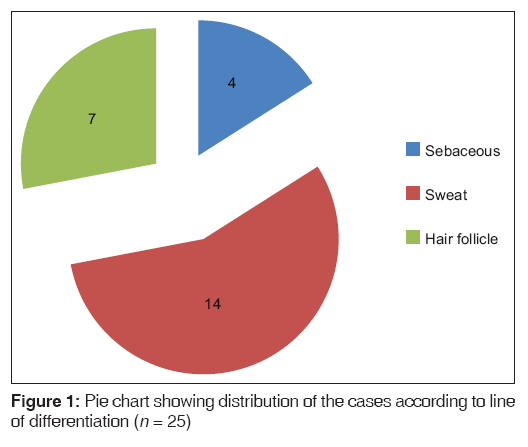
In the present study, over a period of 4 years, a total of 25 ATs of the skin were diagnosed. Benign ATs constituted the majority of tumors with 24 out of 25 cases (96%) while only one case of malignant AT was encountered. The sweat gland tumors accounted for the largest number of cases 56% (14/25), followed by hair follicle tumors (28%) and sebaceous gland tumors (16%) [Figure 1].
The male-to-female ratio was approximately equal (0.92:1). The age of the patients’ ranged from 8 years to 58 years, with a median age of 32 years. The highest incidence of ATs was encountered in 21–40 years’ age group (56%).
The head and neck region was the most frequently involved location for ATs of the skin accounting for 72% of the cases followed by extremities (20%) and trunk (8%). Clinically, these patients were suspected to have cysts, soft tissue tumors, or ATs. In 48% of patients (12/25), ATs were clinically suspected by the clinician before excision, while in 52% of patients, ATs were diagnosed only on histopathology. The tumor size ranged from 0.5 cm to 5 cm in maximum dimension, with 80% of the tumors being <2.5 cm in diameter. The clinicopathological characteristics of the study group are shown in Table 1.
| Characteristics | Total (n=25), n (%) |
|---|---|
| Age (years) | |
| 0-10 | 1 (4) |
| 11-20 | 3 (12) |
| 21-30 | 7 (28) |
| 31-40 | 7 (28) |
| 41-50 | 5 (20) |
| >50 | 2 (8) |
| Sex | |
| Males | 12 (48) |
| Females | 13 (52) |
| Location | |
| Head and neck | 18 (72) |
| Extremities | 5 (20) |
| Trunk | 2 (8) |
| Size (maximum dimension in cm) | |
| <1.5 | 13 (52) |
| 1.6-2.5 | 7 (28) |
| 2.6-4.0 | 4 (16) |
| >4.1 | 1 (4) |
| Differentiation | |
| Sebaceous | 4 (16) |
| Sweat | 14 (56) |
| Hair follicle | 7 (28) |
| Behavior | |
| Benign | 24 (96) |
| Malignant | 1 (4) |
Table 1: Clinicopathological characteristics of the study group
The most common varieties of tumors encountered in the present study included hidradenoma papilliferum and pilomatricoma with four cases each. The frequency distribution of the various adnexal skin tumors is shown in Table 2.
| Tumor | Category | n (%) |
|---|---|---|
| Hidradenoma papilliferum | Sweat gland | 4 (16) |
| Pilomatricoma | Hair follicle | 4 (16) |
| Apocrine hidradenoma | Sweat gland | 2 (8) |
| Proliferating trichilemmal tumor | Hair follicle | 2 (8) |
| Cylindroma | Sweat gland | 2 (8) |
| Chondroid syringoma | Sweat gland | 2 (8) |
| Nevus sebaceous | Sebaceous gland | 2 (8) |
| Eccrine poroma | Sweat gland | 1 (4) |
| Syringoma | Sweat gland | 1 (4) |
| Sebaceous hyperplasia | Sebaceous gland | 1 (4) |
| Sebaceous carcinoma | Sebaceous gland | 1 (4) |
| Trichoepithelioma | Hair follicle | 1 (4) |
| Nodular hidradenoma | Sweat gland | 1 (4) |
| Syringocystadenoma papilliferum | Sweat gland | 1 (4) |
Table 2: The frequency distribution of the various adnexal skin tumors
Discussion
The benign tumors of epidermal appendages can be classified into four groups depending on the line of differentiation into tumors with differentiation toward hair follicles, toward sebaceous glands, toward apocrine glands, and toward eccrine glands. The malignant ATs are of three major types: carcinoma of sebaceous glands, of eccrine glands, and of apocrine glands. Occasional cases of malignant tumors of hair follicle differentiation have also been reported such as pilomatrical carcinoma, malignant proliferating trichilemmal cyst, and trichilemmal carcinoma.[1,2] The incidence of benign ATs outnumbers the malignant ones in most of the studies in literature.[4-17] In the present study also, 96% (24/25) of the tumors were benign with only a single malignant tumor.
The diagnosis of ATs poses great diagnostic difficulties owing to a variety of reasons, namely, the enormous types of tumor with their variants, the occurrence of multiple lines of differentiation in a single tumor as well as the complicated nomenclature. ATs originate from multipotential undifferentiated stem cells, rather than mature cells, which have the capability to differentiate along particular pathways, maybe multiple.[1,2]
Among the ATs, the most frequent line of differentiation encountered was sweat gland differentiation (56%), followed by hair follicle differentiation (28%) and the least frequent being sebaceous gland differentiation (16%). These observations are in concordance with that of Radhika et al.,[14] Sharma et al.,[17] Pantola et al.,[15] and Gayathri et al.[13] On the contrary, Kamyab-Hesari et al.[16] found sebaceous tumors to be the most common type which is unlike most of the studies in literature. A comparative analysis of many studies from Indian literature has been shown in Table 3 with regard to the line of differentiation of ATs.
| Study | Year | Number of | Sebaceous differentiation, | Sweat gland differentiation, | Hair follicle differentiation, |
|---|---|---|---|---|---|
| cases (n) | n (%) | n (%) | n (%) | ||
| Vaishnav and Dharkar[4] | 1974 | 48 | 3 (6.3) | 38 (79.2) | 6 (12.5) |
| Kartha et al.[5] | 1980 | 82 | 3 (3.66) | 45 (54.88) | 35 (42.7) |
| Reddy et al.[6] | 1982 | 85 | 18 (21.18) | 54 (63.53) | 13 (15.3) |
| Nair[9] | 2008 | 33 | 2 (6.06) | 19 (58.1) | 12 (36.36) |
| Saha et al.[11] | 2011 | 23 | 3 (13) | 13 (57) | 7 (30) |
| Jindal and Patel[12] | 2012 | 25 | 1 (4) | 13 (52) | 11 (44) |
| Gayathri et al.[13] | 2012 | 29 | 3 (10.3) | 15 (51.72) | 11 (37.93) |
| Radhika et al.[14] | 2013 | 35 | 7 (20) | 17 (48.57) | 11 (31.43) |
| Pantola et al.[15] | 2013 | 70 | 4 (5.7) | 42 (60) | 24 (34.3) |
| Sharma et al.[17] | 2014 | 56 | 12 (21.43) | 24 (42.86) | 20 (35.71) |
| Present study | 25 | 4 (16) | 14 (56) | 7 (28) |
Table 3: A comparative analysis of Indian literature with regard to the line of differentiation of adnexal tumors
The male-to-female ratio of the patients was found to be approximately equal (0.92:1) in our study group, which is comparable to that of Sharma et al.,[17] Kamyab-Hesari et al.,[16] and Jindal and Patel[12] Several other researchers obtained variable results, namely, Nair et al.[9] found male-to-female ratio of 1:2.3 while Pantola et al.[15] found the ratio of 1.8:1.
In the Indian population, the overall incidence of skin ATs is very low as documented by several studies.[9,11-15,17] The location of ATs varies with the histologic type. By far, head and neck region is the most common location where ATs are frequently encountered, the other sites being axilla, trunk, legs, etc. In the present study, in 72% of the cases, the site of predilection was head and neck followed by extremities (20%) and trunk (8%). The predominance of ATs in the head and neck region is a well-documented fact supported by most of the series in literature. This is accounted for by the fact that this region is rich in pilosebaceous units, apocrine as well as eccrine glands, thereby providing a fertile environment for the development of ATs.[18]
Most of the ATs in our study (52%) were <1.5 cm in maximum dimensions. Very rarely, the size of these tumors exceeds 4 cm in diameter. Jindal and Patel[12] also observed that 76% of tumors in their study group were <2 cm in size.
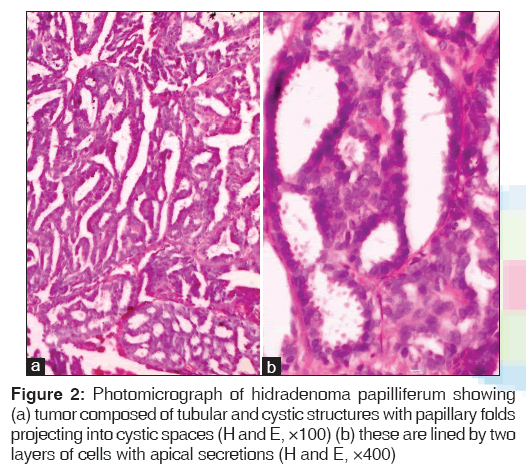
Hidradenoma papilliferum was the most commonly encountered tumor in our series with a total of four cases. It is usually found in women in labia majora or in perineal or perianal region. We came across an interesting case of a cystic swelling in the right labia minora in which the clinical impression was a Bartholin’s cyst. It is a well-circumscribed tumor located in dermis composed of tubular and cystic structures with papillary folds projecting into cystic spaces [Figure 2].
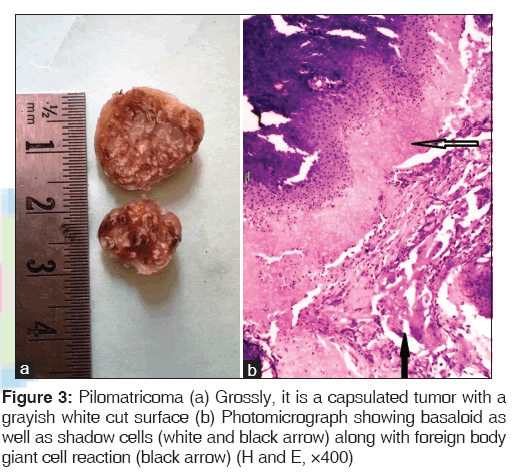
We also came across four cases of pilomatricoma or calcifying epithelioma of Malherbe. It is a tumor with differentiation toward hair follicles, usually seen as a solitary lesion in the face and upper extremities. Microscopically, islands of epithelial cells of two types are seen: basophilic cells and shadow cells [Figure 3].[1,2]
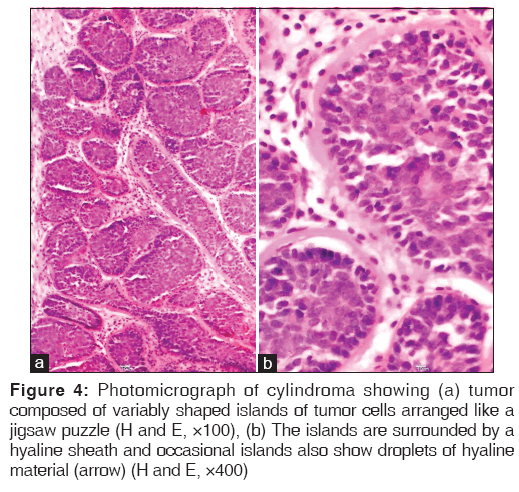
Cylindroma is a tumor where the differentiation in most cases is toward apocrine structures but rarely toward eccrine structures. The tumor is composed of variably shaped islands of tumor cells arranged like a jigsaw puzzle. The islands are surrounded by a hyaline sheath and occasional islands also show droplets of hyaline material [Figure 4]. PAS stain was done to highlight the eosinophilic basement membrane material surrounding the nests of cells in the cases encountered in this study.
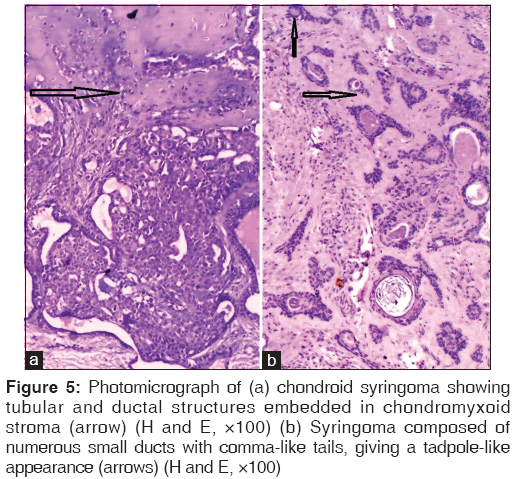
Chondroid syringoma, also known as benign mixed tumor of the skin, is an uncommon adnexal neoplasm that usually occurs on the head and neck as in the present series. Tubular or ductal structures of either eccrine or apocrine type are loosely embedded in a myxoid chondroid matrix in the mid-to-deep dermis. The stroma was intensely positive for alcian blue stain in one of the cases. Syringoma represents an adenoma of intraepidermal eccrine ducts. These are usually multiple, small skin-colored papules located usually on the lower eyelids, cheeks, axillae, etc. The tumor is composed of numerous small ducts lined by two layers of epithelial cells. Some of the ducts have comma-like tails, giving a tadpole-like appearance [Figure 5].[1]
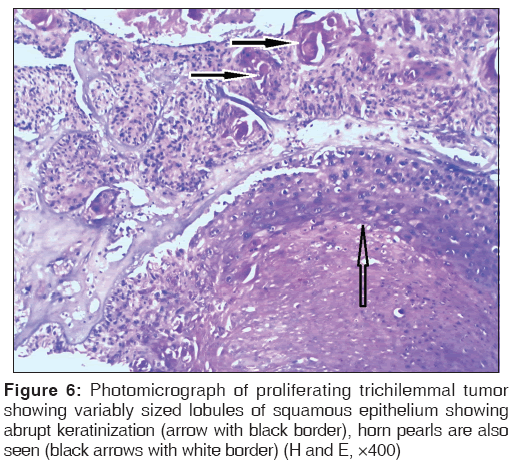
Proliferating trichilemmal tumor is almost always located on the scalp as a solitary lesion. Microscopically, it is a well-circumscribed tumor comprising variably sized lobules of squamous epithelium showing abrupt keratinization. At places, horn pearls are also seen [Figure 6].
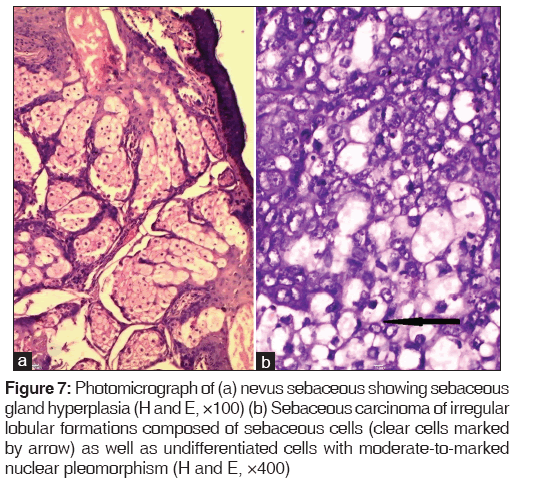
The only malignant tumor encountered in our series was sebaceous carcinoma. It occurs most frequently on the eyelids as seen in our case. The tumor is composed of irregular lobular formations composed of sebaceous cells as well as undifferentiated cells with moderate-to-marked nuclear pleomorphism [Figure 7].[1]
Figure 7: Photomicrograph of (a) nevus sebaceous showing sebaceous gland hyperplasia (H and E, ×100) (b) Sebaceous carcinoma of irregular lobular formations composed of sebaceous cells (clear cells marked by arrow) as well as undifferentiated cells with moderate-to-marked nuclear pleomorphism (H and E, ×400)
The diagnosis of ATs is not suspected clinically in most of the cases. ATs of the skin present either as asymptomatic papules or nodules, which are suspected to be either keratinous cysts or soft tissue nodules. Hence, the discrepancy between clinical and histopathological diagnosis is often encountered. However, the location, single/multiple lesions, and their distribution can provide important diagnostic clues. In the present study, the concordance between clinical and histopathological diagnosis was found to be 50% approximately. Kamyab-Hesari et al.[16] found a slightly higher (64%) concordance between clinical and histopathological diagnoses. This further stresses upon the fact that histopathology still remains the gold standard for the diagnosis of ATs as the clinical presentation is quite indistinctive. Cytochemical stains such as PAS, mucicarmine, alcian blue, and reticulin may aid in establishing the diagnosis. PAS stain highlights the characteristic eosinophilic globules in a spiradenoma as well as the basement membrane material surrounding the nests of tumor cells and small round droplets in a case of cylindroma. Alcian blue stain helps in demonstrating the intense staining of the stroma in a case of chondroid syringoma.[1,2]
Immunohistochemistry (IHC) has little, if any, role in differentiating between the various types of ATs. Carcinoembryonic antigen positivity indicates a ductal differentiation, cytokeratin (CK) indicates follicular differentiation while sebaceous differentiation is suggested by positive epithelial membrane antigen. IHC may play an important role in distinguishing primary cutaneous adnexal carcinoma from metastatic carcinoma. P63 and CK 5/6 positivity favors a primary cutaneous adnexal carcinoma over a metastatic carcinoma.[1,2]
Conclusion
ATs of the skin are rare neoplasms with benign tumors being far more common. They are often misdiagnosed clinically, so histopathology remains the gold standard for establishing an accurate diagnosis of skin ATs.
Financial support and sponsorship
Nil.
Conflict of Interest
There are no conflicts of interest.
REFERENCES
- Klein W, Chan E, Seykora JT. Tumors of the epidermal appendages. In: Elder DE, Elenitsas R, Johnson BL Jr., Murphy GF, editors. Lever’s Histopathology of the Skin. 9th ed. Philadelphia: Lippincott Williams and Wilkins; 2005.867-926.
- Santa Cruz DJ. Tumors of the skin. In: Fletcher CD, editor. Diagnostic Histopathology of Tumors. 3rd ed., Vol. 2. Philadelphia: Churchill Living Stone Elsevier; 2007. 1423-526.
- Kanitakis J. Adnexal tumours of the skin as markers of cancer-prone syndromes. J Eur Acad Dermatol Venereol 2010;24:379-87.
- Vaishnav VP, Dharkar DD. Adnexal tumours of skin. Indian J Pathol Bacteriol 1974;17:33-8.
- Kartha CC, Shankar SK, Bhuyan UN. Benign mixed tumour of skin – A histopathologic study of 7 cases. Indian J Pathol Microbiol 1980;23:1-6.
- Reddy MK, Veliath AJ, Nagarajan S, Aurora AL. A clinicopathological study of adnexal tumours of skin. Indian J Med Res 1982;75:882-9.
- Wong TY, Suster S, Cheek RF, Mihm MC Jr. Benign cutaneous adnexal tumors with combined folliculosebaceous, apocrine and eccrine differentiation: Clinicopathological and immunohistochemical study of eight cases. Am J Dermatopathol 1996;18:124-8.
- Yaqoob N, Ahmad Z, Muzaffar S, Gill MS, Soomro IN, Hasan SH. Spectrum of cutaneous appendage tumors at Aga Khan University Hospital. J Pak Med Assoc 2003;53:427-31.
- Nair PS. A clinicopathologic study of skin appendageal tumors. Indian J Dermatol Venereol Leprol 2008;74:550.
- Samaila MO. Adnexal skin tumors in Zaria, Nigeria. Ann Afr Med 2008;7:6-10.
- Saha A, Das NK, Gharami RC, Chowdhury SN, Datta PK. A clinico-histopathological study of appendageal skin tumors, affecting head and neck region in patients attending the dermatology opd of a tertiary care centre in Eastern India. Indian J Dermatol 2011;56:33-6.
- Jindal U, Patel R. Study of adnexal tumors of the skin: A three year study of 25 cases. Int J Pathol 2012;13:3.
- Gayathri SS, Alavandar E, Kumar SA. An analysis of skin appendageal tumors in South India. J Evol Med Dent Sci 2012;1:907-10.
- Radhika K, Phaneendra BV, Rukmangadha N, Reddy MK. A study of biopsy confirmed skin adnexal tumours: Experience at a tertiary care teaching hospital. J Clin Sci Res 2013;2:132-8.
- Pantola C, Kala S, Agarwal A, Amit S, Pantola S. Cutaneous adnexal tumours: A clinicopathological descriptive study of 70 cases. World J Pathol 2013;2:77-82.
- Kamyab-Hesari K, Balighi K, Afshar N, Aghazadeh N, Rahbar Z, Seraj M, et al. Clinicopathological study of 1016 consecutive adnexal skin tumors. Acta Med Iran 2013;51:879-85.
- Sharma A, Paricharak DG, Nigam JS, Rewri S, Soni PB, Omhare A, et al. Histopathological study of skin adnexal tumours-Institutional study in South India. J Skin Cancer 2014;4:543756.
- Burns T, Breathnach S, Cox N, Griffiths C. Tumors of the skin appendages. In: Mackie RM, Calonje E, editors. Rook’s Textbook of Dermatology. 7th ed. London: Blackwell Publishing Company; 2004. p. 1-34.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.