Age and Gender Risk factors for Macular Degeneration: Systematic Review and Meta-analysis
2 General Practitioner at Khamis Mushayt General Hospital, Abha City, Saudi Arabia
3 Service Resident at King Abdulaziz Specialist Hospital, Taif City, Saudi Arabia
4 Medical Student at King Khalid University, Abha City, Saudi Arabia
5 Medical Intern, Ibn Sina National College, Jeddah, Saudi Arabia
6 General Practitioner, Jordan University of Science and Technology, Riyadh City, Saudi Arabia
Citation: Mohammed MA, et al. Age and Gender Risk factors for Macular Degeneration: Systematic Review and Meta- analysis. Ann Med Health Sci Res. 2021;11: 1188-1192.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: AMD is considered a multifactorial disease associated with genetic and environmental factors. Age is the strongest non-modifiable risk factor. The risk of developing advanced AMD is 3 times higher among individuals aged 60-80 years than in those under the age of 60.5 Aim: This work aims to determine the effect of age and gender as risk factors in Macular Degeneration (MD) patients. Materials and Methods: A systematic search was performed over different medical databases to identify Ophthalmology studies, which studied the outcome of MD patients. Using the meta-analysis process, either with fixed or random-effects models, we conducted a meta-analysis on the overall MD prevalence as a primary outcome, and no correlation between age, gender, and MD prevalence as secondary outcomes. Results: Seven studies were identified involving 30515 patients, 14790 in the Male group and 15725 in the Female group. The meta-analysis process revealed that the pooled MD prevalence of (11.8%), with a non-significant difference in MD prevalence in the Male group, compared to the Female group (p > 0.05). Using Spearman’s correlation analysis, the meta-analysis process revealed a highly significant positive correlation between age and MD prevalence (p < 0.05). Conclusion: To conclude, age-related macular degeneration (AMD) is a leading cause of blindness in the elderly. The lesions associated with AMD are commonly divided into early AMD (soft drusen with or without retinal pigment abnormalities, frequently called “age-related maculopathy”) and late AMD (neovascular disease and geographic atrophy). High age and early AMD are strong risk factors for developing late AMD.
Keywords
Risk factors; Age; Macular degeneration
Introduction
Age-related Macular Degeneration (AMD) became firstly described in 1885 by Otto Haab as a disease that was characterized by pigmented and atrophic changes in macula and progressive central vision loss in patients over 50. The other call of the disease is senile macular degeneration. AMD is chronic and progressive disorder and in advanced countries, it’s far one of the major reasons for irreversible crucial vision loss in the population of over 50. In 2030, its miles expected that AMD might be the main reason for blindness by using leaving behind diabetic retinopathy (DRP) and glaucoma in developed countries. [1]
With longer life expectancy, age-related disorders are increasing the burden positioned on fitness care providers. In particular, age-associated macular degeneration (ARMD) is one of the primary causes of vision loss in the elderly. ARMD presently affects 6 million human beings inside the United Kingdom alone and was expected to have cost the country’s economic system £155 million in 2011. By 2040, the number of humans affected globally via the sickness is projected to be 288 million. [2]
Age-associated macular degeneration (AMD) is a leading cause of blindness in the elderly. The lesions associated with AMD are normally divided into early AMD (soft drusen with or without retinal pigment abnormalities, often referred to as “age-associated maculopathy”) and late AMD (neovascular ailment and geographic atrophy). High age and early AMD are robust risk elements for growing past due AMD. With increasing longevity, the weight of age-associated sicknesses will boom. Identification of humans at the chance for late AMD and early treatment of neovascular AMD is important to prevent rapid visual loss. Maintenance of visible features is critical for excellent lifestyles and diminishes the need for domestic services. For that reason, estimates of AMD rates from any given population are critical for planning eye fitness offerings and rehabilitation. [3]
Today, AMD is considered a multifactorial disease associated with genetic and environmental factors. Age is the strongest non-modifiable risk factor. The risk of developing advanced AMD is 3 times higher among individuals aged 60-80 years than in those under the age of 60.5. Smoking is another important but modifiable risk factor. Many studies have demonstrated the impact of smoking on AMD development and report that smokers are likely to develop AMD 5-10 years earlier than nonsmokers. [4]
This work aims to determine the effect of age and gender as risk factors in macular degeneration patients.
Literature Review
Our review came following the (PRISMA) statement guidelines. [5]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing MD patients. The excluded studies were non-English, or animal studies, or describing other types of MD patients (e.g. drug-induced MD, diabetic macular degeneration).
Study identification
Basic searching was done over the PubMed, Cochrane library, and Google scholar using the following keywords: Risk factors, Age, Macular Degeneration.
Data extraction and synthesis
RCTs, clinical trials, and comparative studies, which studied the outcome of Male group versus Female group of MD patients, will be reviewed. Outcome measures included the overall MD prevalence as a primary outcome, and the correlation between age, gender, and MD prevalence as secondary outcomes.
Study selection
We found 249 records, 200 excluded based on title and abstract review; 49 articles are searched for eligibility by full-text review; 19 articles cannot be accessed; 15 studies were reviews and case reports; 8 were not describing functional outcome; leaving 7 studies that met all inclusion criteria.
Statistical analysis
After the pooling of data, Odds ratios (OR), Proportions (%), with 95% confidence intervals (CI) were calculated, using MedCalc statistical software (Belgium). After the Q test of heterogeneity, the I2-statistics (either the fixed-effects model or the random-effects model) were done within the meta-analysis process.
Results
The included studies were published between 2011 and 2020 [Table 1]. [3,6-11] Regarding patients’ characteristics, the total number of patients in all the included studies was 30515 patients, 14790 in the Male group and 15725 in the Female group, while their average age was (64.6 years) [Table 1].
A meta-analysis study was done on 7 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=30515) [Table 2]. [3,6-11]
| S.No | Author | Year of publication | Number of patients | Age (Average years) |
||
|---|---|---|---|---|---|---|
| Total | Male group | Female group | ||||
| 1 | Lim et al. [6] | 2011 | 14352 | 6799 | 7553 | 64.7 |
| 2 | Erke et al. [3] | 2012 | 2631 | 1512 | 1119 | 72.3 |
| 3 | Ueda-Arakawa et al. [7] | 2013 | 216 | 161 | 55 | 74.8 |
| 4 | Korb et al. [8] | 2014 | 4340 | 2164 | 2176 | 55.5 |
| 5 | Brandl et al. [9] | 2016 | 2546 | 1278 | 1268 | 47.5 |
| 6 | Wilde et al. [10] | 2017 | 3475 | 1536 | 1939 | 75 |
| 7 | Behboudi et al. [11] | 2020 | 2955 | 1340 | 1615 | 62.6 |
| #Studies arranged via publication year. | ||||||
Table 1: Patients and study characteristics.
| S. No | Author | Primary outcome | Secondary outcome | |
|---|---|---|---|---|
| Overall MD prevalence |
MD prevalence | |||
| Male group | Female group | |||
| 1 | Lim et al. [6] | 865 | 357 | 508 |
| 2 | Erke et al. [3] | 99 | 26 | 73 |
| 3 | Ueda-Arakawa et al. [7] | 97 | 76 | 21 |
| 4 | Korb et al. [8] | 616 | 324 | 292 |
| 5 | Brandl et al. [9] | 283 | 139 | 144 |
| 6 | Wilde et al. [10] | 255 | 102 | 153 |
| 7 | Behboudi et al. [11] | 316 | 125 | 191 |
Table 2: Summary of outcome measures in all studies.
Each outcome was measured by:
Prevalence (%)
• For overall MD prevalence.
Odds Ratio (OR)
• For gender and MD prevalence.
Correlation analysis
• For correlation between age and MD prevalence.
Concerning the primary outcome measure, we found 7 studies reported MD prevalence with a total number of patients (N=30515). I2 (inconsistency) was 98.9% with a highly significant Q test for heterogeneity (p<0.0001), so randomeffects model was carried out; with overall prevalence = 11.8% (95% CI = 8.213 to 16.015).
Using the random-effects model, the meta-analysis process revealed a pooled MD prevalence of (11.8%) (p<0.05) [Figure 1].
Concerning the secondary outcome measures, we found 7 studies reported gender and MD prevalence.
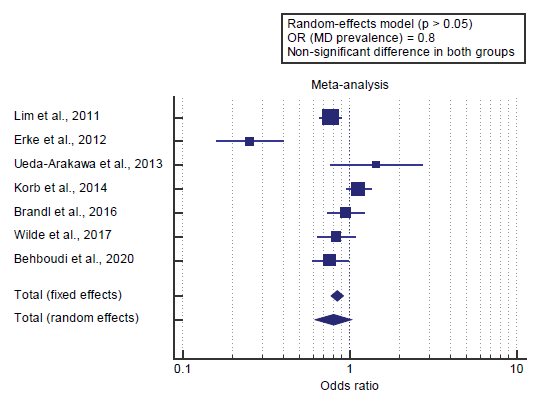
I2 (inconsistency) was 86.7% with a highly significant Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with overall OR= 0.8 (95% CI = 0.618 to 1.028).
Using the random-effects model, the meta-analysis process revealed a non-significant difference in MD prevalence in the Male group compared to the Female group (p>0.05) [Figure 2].
We found 7 studies reported age and MD prevalence. Using Spearman’s correlation analysis, the meta-analysis process revealed a highly significant positive correlation between age and MD prevalence (p<0.05) [Figure 3].
Discussion
This work aims to determine the effect of age and gender as risk factors in macular degeneration patients. The included studies were published between 2011 and 2020. Regarding patients’ characteristics, the total number of patients in all the included studies was 30515 patients, 14790 in the Male group and 15725 in the Female group, while their average age was (64.6 years).
A meta-analysis study was done on 7 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=30515). Concerning the primary outcome measure, we found 7 studies reported MD prevalence with a total number of patients (N=30515).
Using the random-effects model, the meta-analysis process revealed a pooled MD prevalence of (11.8%) (P<0.05). Which came in agreement with Pead et al., [2] Sengul et al., [12] Erke et al. [3] and Dougherty et al. [13]
Pead et al. reported that the rising incidence of age-related eye diseases, specifically age-associated macular degeneration, places an ever-growing burden on health care providers. As new remedies emerge, it is important to expand methods for reliably assessing sufferers’ sickness status and stratifying risk of progression. [2]
Sengul et al. reported that age-associated macular degeneration (AMD) is a leading cause of vision loss inside the aged worldwide. Even though its pathogenesis has now not been elucidated fully, vascular endothelial growth aspect (VEGF) has been shown to play a key role in the improvement of choroidal neovascularization, which can cause severe vision loss if left untreated. [12] Erke et al. reported that the present cross-sectional study described the superiority rates of AMD among Caucasian elderly participants from the Tromsø Eye Study, a populationbased study in Norway. The overall prevalence of past-due AMD becomes 3.5%, and neovascular disorder outnumbered geographic atrophy. Symmetry among eyes turned into highly low. [3]
Dougherty et al. reported that most research supplied crude AMD prevalence estimates, while some best-provided rates have been adjusted to an intended study population or a national trendy. AMD prevalence prices numerous broadly and ranged from 1.1% in EDPRG to 40.5% within the have a look at of Osteoporotic Fractures. [13]
Concerning the secondary outcome measures, we found 7 studies reported gender and MD prevalence. Using the random-effects model, the meta-analysis process revealed a non-significant difference in MD prevalence in the Male group compared to the Female group (p>0.05), which came in agreement with Erke et al. [3]
Erke et al. reported that the proportion of late AMD cases with bilateral involvement increased from 14% in participants aged 65 to 69 years to 67% in participants elderly 80 to 87 years. There has been no significant sex distinction in typical symmetry. The symmetry between eyes turned exceptionally low. Incidence accelerated strongly with age. No vast sex variations in incidence rates of AMD have been located. Refractive error turned into lower in eyes with past due to AMD than in eyes without overdue AMD. No universal sex variations in the prevalence of huge drusen >125 μm or overdue AMD were observed in the present examination. This is regular with studies from Western populations and contradicts the findings from Asian populations in whom a higher occurrence of drusen and overdue AMD became observed in guys than in ladies. Our findings of an increased occurrence of large drusen and late AMD with age have been much like those of previously published studies. [3]
We found 7 studies reported age and MD prevalence. Using Spearman’s correlation analysis, the meta-analysis process revealed a highly significant positive correlation between age and MD prevalence (p<0.05). Which came in agreement with Janot et al., [14] Pead et al., [2] Yurtseven et al. [4] Dougherty et al. [13] Horie-Inoue & Inoue [15] and Erke et al. [3]
Janot et al. reported that, through multivariate regression analysis, having ever smoked cigarettes (OR=5.24, p=0.007), male sex (OR=7.48, p=0.002) were found to be independent risk factors, and age at chart review was found to be a concomitant risk factor (OR= 1.11, p=0.002) for the development of MD. [14]
Pead et al. reported that, with longer life expectancy, agerelated disorders are increasing the burden placed on health care providers. In particular, age-related macular degeneration (ARMD) is one of the major causes of vision loss in the elderly. ARMD currently affects 6 million people in the UK alone and was estimated to have cost the country’s economy £155 million in 2011. By 2040, the number of people affected globally by the disease is projected to be 288 million. [2]
Yurtseven et al. reported that AMD is considered a multifactorial disease associated with genetic and environmental factors. Age is the strongest non-modifiable threat factor. The hazard of growing advanced AMD is 3 times better amongst individuals elderly 60-eighty years than in those underneath the age of 60.5 Smoking is any other important however modifiable hazard issue. Many studies have validated the impact of smoking on AMD development and document that smokers are possibly to broaden AMD five-10 years in advance than non-smokers. [4]
Dougherty et al. reported that many studies provided results stratified by age, race/ethnicity, and sex. But, age and race/ ethnicity classifications differed across studies: a few study populations included one race/ethnicity category, whilst others included or 3 racial and ethnic subgroups. Further, age categories varied across studies as did the included age groups: some studies included adults 18 years and older, whilst others included individuals elderly 40 or sixty-five years or older. The data provided for each look in this section focus on differences by age, sex, and race/ethnicity. [13]
Horie-Inoue & Inoue reported that the racial differences in AMD occurrence might not be explained even after controlling for known non-genetic hazard factors including body mass index, smoking, and alcohol drinking history, diabetes and hypertension status. It’s also notable that the frequency of exudative AMD was maximum in Chinese with age- and gender-adjusted odds ratio as 4.3 compared with whites. [15]
Erke et al. reported that the prevalence of late AMD improved significantly with age (OR per five to twelve months increase 2.32). No sex difference became found adjusted for age. At some point in the interview, as much as 52% of late AMD instances denied having been identified with AMD. Women with late AMD had a higher frequency of self-reported cataract (OR, 6.95). [3]
Conclusion
To conclude, age-related macular degeneration (AMD) is a leading cause of blindness in the elderly. The lesions associated with AMD are commonly divided into early AMD (soft drusen with or without retinal pigment abnormalities, frequently called “age-related maculopathy”) and late AMD (neovascular disease and geographic atrophy). High age and early AMD are strong risk factors for developing late AMD.
Competing Interests
The authors declare that they have no competing interests. All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
REFERENCES
- Simsek B, Yaylali SA. The effectiveness of intravitreal aflibercept in the treatment of neovascular age-related macular degeneration. Population 2014.
- Pead E, Megaw R, Cameron J, Fleming A, Dhillon B, Trucco E, et al. Automated detection of age-related macular degeneration in color fundus photography: a systematic review. Surv Ophthalmol. 2019;64:498-511.
- Erke MG, Bertelsen G, Peto T, Sjølie AK, Lindekleiv H, Njølstad I. Prevalence of age-related macular degeneration in elderly Caucasians: the Tromsø Eye Study. Ophthalmol. 2012;119:1737-1743.
- Yurtseven ÖG, Aksoy S, Arsan AK, Özkurt YB, Kökçen HK. Evaluation of the Relationship Between Age-related Macular Degeneration and Refractive Error, Socio-demographic Features, and Biochemical Variables in a Turkish Population. Turk J Ophthalmol. 2018;48:238.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Bmj 2009;339.
- Lim S, Shin H, Song JH, Kwak SH, Kang SM, Yoon JW, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care. 2011;34:1323-1328.
- Ueda-Arakawa N, Ooto S, Nakata I, Yamashiro K, Tsujikawa A, Oishi A, et al. Prevalence and genomic association of reticular pseudodrusen in age-related macular degeneration. Am J Ophthalmol. 2013;155:260-269.
- Korb CA, Kottler UB, Wolfram C, Hoehn R, Schulz A, Zwiener I, et al. Prevalence of age-related macular degeneration in a large European cohort: results from the population-based Gutenberg Health Study. Graefes Arch Clin Exp Ophthalmol. 2014;252:1403-1411.
- Brandl C, Breinlich V, Stark KJ, Enzinger S, Aßenmacher M, Olden M, et al. Features of age-related macular degeneration in the general adults and their dependency on age, sex, and smoking: results from the German KORA study. PloS One 2016;11:e0167181.
- Wilde C, Poostchi A, Mehta RL, MacNab HK, Hillman JG, Vernon SA, et al. Prevalence of age-related macular degeneration in an elderly UK Caucasian population—The Bridlington eye assessment project: a cross-sectional study. Eye 2017;31:1042-1050.
- Behboudi H, Nikkhah H, Alizadeh Y, Katibeh M, Pakbin M, Ahmadieh H, et al. A Population-based Study on the Prevalence and Associated Factors of Age-related Macular Degeneration in Northern Iran the Gilan Eye Study. Ophthalmic Epidemiol. 2020;27:209-218.
- Sengul EA, Artunay O, Kumral ET, Yenerel M, Rasier R, Kockar A, et al. Retinal nerve fiber layer thickness changes in age-related macular degeneration treated with multiple intravitreal ranibizumab. J Ocul Pharmacol Ther. 2016;32:665-670.
- Dougherty M, Wittenborn J, Phillips E, Swenor B. Published Examination-based Prevalence of Major Eye Disorders 2018.
- Janot AC, Huscher D, Walker M, Grewal HK, Yu M, Lammi MR, et al. Cigarette smoking and male sex are independent and age concomitant risk factors for the development of ocular sarcoidosis in a New Orleans sarcoidosis population. Sarcoidosis, Vasculitis, and Diffuse Lung Diseases: Official Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:138.
- Horie-Inoue K, Inoue S. Genomic aspects of age-related macular degeneration. Biochem Bioph Res Co. 2014;452:263-275.






 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.