Antimicrobial Synergic Effect of Allicin and Silver Nanoparticles on Skin Infection Caused by Methicillin‑Resistant Staphylococcus aureus spp
- *Corresponding Author:
- Seyedeh Mahsan Hoseini- Alfatemi
Department of Bacteriology and Virology, Shiraz Medical School, Shiraz University of Medical Sciences, Shiraz, Iran.
E-mail: m.hoseinialfatemi@gmail.com
Citation: Sharifi-Rad J, Hoseini Alfatemi SM, Sharifi Rad M, Iriti M. Antimicrobial synergic effect of allicin and silver nanoparticles on skin infection caused by methicillin-resistant staphylococcus aureus spp. Ann Med Health Sci Res 2014;4:863-8.
Abstract
Background: Today, the commonly used antibiotics may more and more frequently be ineffective against multiple pathogens, due to the selection of resistant microbial strains. As a result, an effort to find a new approach for solving this issue has been considered. Aim: The aim of this study is to investigate antimicrobial properties of allicin, silver nanoparticles (Ag NPs) and their combination again skin infection caused by methicillin‑resistant Staphylococcus aureus (MRSA) strains in an animal model. Materials and Methods: In vivo, the effects of allicin, Ag NPs and their combination were investigated on mice in which the skin infection was caused by MRSA strains. In animals, S. aureus colony‑forming units (CFU)/mL were counted the 4th day after treatment. In vitro, minimum inhibitory concentration (MIC) of bacterial growth and minimum bactericidal concentration (MBC) of allicin, Ag NPs and their combination were determined by microdilution technique. Results: The results of in vitro assays showed that MIC of allicin and Ag NPs were 2.2 mg/mL and 5.6 mg/mL, respectively, and MBC of allicin and Ag NPs were 3.1 ppm and 7.5 ppm, respectively. However, MIC and MBC of allicin and Ag NPs together on MRSA strains were 0.4 mg/mL and 1.1 ppm, respectively. The results of in vivo tests on skin infection showed that bacteria counted for control, Ag NPs, allicin and their combination were 377 × 108, 80 × 106, 43 × 105, and 0 CFU/mL, respectively. Conclusion: The obtained results clearly indicated (for the first time, to the best of our knowledge) that allicin and Ag NPs, when used in combination, exhibited a synergistic activity. Therefore, the present results can be of interest in the future to improve the treatment of skin infections caused by MRSA strains.
Keywords
Allicin, Antimicrobial synergic activity, Methicillin‑resistant Staphylococcus aureus spp., Silver nanoparticles, Skin infection
Introduction
Staphylococcus bacteria are Gram‑positive bacteria arranged in the form of regular clusters. Some of them are components of the normal flora of human skin and mucosa, sometimes causing the production of pus and abscesses, fatal septicemia or suppurative infections. Staphylococcus aureus is a major human pathogen responsible for a significant number of nosocomial and community‑associated infections throughout the world.[1‑4] Today, the improper use of antibiotics has been causing the emergence of antibiotic‑resistant bacteria, including methicillin‑resistant S. aureus (MRSA) strains.[4] In these bacteria, resistance to methicillin is due to the mecA gene, included in the staphylococcal cassette chromosome mec.[5] The mecA gene encodes a secondary protein of 78 kD called penicillin‑binding protein, which, in the presence of β‑lactam antibiotics, prevent them from inhibiting bacterial cell wall biosynthesis.[6]
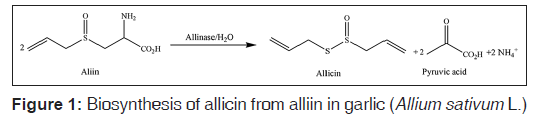
Plants as a resource of natural remedies have been considered for the treatment of diseases. Antibacterial activity is one of the main properties of plants. In addition, antimicrobial compounds from plants may be effective in counteracting infections caused by antibiotic‑resistant bacterial strains. Garlic (Allium sativum L.), belonging to the Alliaceae family, is used for medicinal purposes since thousands of years. Many health benefits have been mentioned for garlic, such as the treatment of arthralgia, prevention of cardiovascular diseases, headache, tuberculosis, leprosy, epilepsy, cough, and digestive disorders.[7‑9] It also showed antimicrobial effects again many viruses, bacteria, fungi and parasites.[10,11] Some medicinal properties of garlic have been attributed to organosulfur compounds, that is, organic compounds that contain sulfur. Allicin (diallyl thiosulfinate) is the most‑important organosulfur compound in garlic.[12] This compound is particularly abundant in garlic, and it is responsible for the typical and pungent odor of garlic. In fresh garlic, allicin derives from alliin (S‑allylcysteine sulfoxide), which is colorless and odorless. When destruction of the cell membrane by crushes or chews occurs, alliin is exposed to the enzyme allinase, which hydrolyzes alliin to an allicin molecule and two disulfide molecules.[13] The biosynthesis of allicin from alliin in garlic is reported in Figure 1.
As previously introduced, herbal remedies have been promoted as alternatives to conventional antimicrobial agents, particularly in the event of antibiotic resistance.[14] Promising in vitro antimicrobial activity again S. aureus was demonstrated for a number of botanical extracts including Salvia officinalis, Eucalyptus globulus, Coleus forskohlii, Arctostaphylos uva‑ursi, Coptis chinensis, Turnera diffusa, Anemopsis californica, Larrea tridentata and A. sativum.[15] Similarly, the methanolic extract of Coriandrum sativum, Crocus sativus and Nerium oleander as well as quinolone alkaloids from fruit of Tetradium ruticarpum exhibited inhibitory activity again MRSA isolates.[16] Interestingly, Aloe vera and Trachyspermum ammi showed antimicrobial activity again MRSA strains isolated in Southeast Iran.[17]
Nanotechnology is widely used in many fields of science and medicine. Exploiting of nanoparticles as antimicrobial agents, in recent years, has been investigated by many researchers. Among the nanoparticles, silver nanoparticles (Ag NPs) have important and significant effects again multidrug‑resistant bacteria.[18] The mechanism of the antibacterial activity of nanoparticles is still poorly understood, and it has been hypothesized that these nanostructures exert their effects on bacterial cell membranes.[18]
Because of the more and more increasing phenomenon of bacterial resistance to antibiotics, in this study, we examined (for the first time, to the best of our knowledge) the in vitro and in vivo antibacterial effects of Ag NPs combined with allicin again MRSA bacterial strains, in order to find new strategies to control antibiotic‑resistant bacteria.
Materials and Methods
Garlic plant preparation
Garlic (A. sativum var. sativum) bulbs were collected from plants grown in Shiraz, Iran. Freshly peeled garlic cloves (400 g) [Figure 2] were frozen in liquid nitrogen and kept at −40°C until analysis.
Sample preparation for high‑performance liquid chromatography analysis
A peeled garlic clove (3-10 g) in 300 mL homogenizing cup was added to 200 mL of 95% methanol solution including 0.01 N HCl and then homogenized, at the highest speed, for 2 min, using a 7012 laboratory blender (Warring Products, Inc., Torrington, CT). The homogenate was decanted into a 300 mL flask. The homogenizing cup was washed with a 95% methanol solution including 0.01 N HCl, and the washing solution was combined with the homogenate to make exactly 300 mL. The mixture was centrifuged at 12,000 rpm for 4 min, and the supernatant was analyzed by high‑performance liquid chromatography (HPLC).
High performance liquid chromatography analysis of allicin
An Agilent 1100 HPLC Series system was used (Agilent Technologies, Darmstadt, Germany), connected with an Agilent Ion Trap SL mass spectrometer (MS) supplied with an electrospray or APCI ion origin. The separation of allicin was carried out utilizing a Synergy Polar 100 × 2.0 mm i.d., 4 μm columns (Phenomenex, SUA). The mobile phase is composed of 100% ammonium acetate, 1 mM in water, isocratic elution, flow 0.6 mL/min. A silver nitrate solution 1 mM in water was added post column, with a flow of 10 μL/min. The MS operated in positive multiple reaction monitoring mode,using an electrospray ion source and nitrogen as nebulizing and dry gas. The nebulizer was set at 60 psi, the dry gas flow was 10 L/min at 355°C. The device was set to record the transition m/z (449 + 451) >m/z (269; 271; 287; 289), specific to allicin‑silver complex. The retention time of allicin in the above described conditions was 0.9 min.[19]
Silver nanoparticles preparation
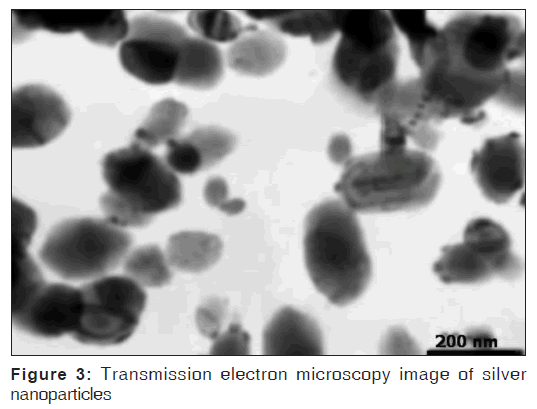
The solution containing Ag NPs was prepared from Nanocid®. The concentration of nanoparticles was 4000 mg/mL, and formulation was in the form of colloidal suspension. The size of nanoparticles was between 10 and 30 nm [Figure 3].
Determination minimum inhibitory concentration and minimum bactericidal concentration of allicin, silver nanoparticles and their combinationDetermination minimum inhibitory concentration and minimum bactericidal concentration of allicin, silver nanoparticles and their combination
The standard strain of MRSA ATCC14458 was used. In vitro minimum inhibitory concentration (MIC) of bacterial growth and minimum bactericidal concentration (MBC) of allicin, Ag NPs and their combination were assessed according to standard microbiological methods in Hinton broth medium.
The MIC values for allicin, Ag NPs and their combination were determined by microplate method of Eloff.[20] All treatments were tested at 9.6 μg/mL[21] and serially diluted two‑fold to 0.1 μg/mL in a 96‑multiwell polystyrene flat‑bottomed microplate (Sigma‑Aldrich, St. Louis, MO, USA); thereafter 100 μL (1 × 106 colony‑forming units [CFU]/mL) of bacteria were added to each well. Pre‑incubation absorbance values were measured from an ELISA reader. The microplates were then incubated overnight at 37°C and absorbance values were measured after 24 h. MIC values were recorded as the lowest concentration of the extract that completely inhibited bacterial growth. In this study, sterility control was medium without allicin, Ag NPs and their combination. Growth control was medium with bacteria but without allicin, Ag NPs and their combination. Bacterial cells from the MIC test plates were sub‑cultured on solid nutrient agar by making streaks on the surface of the agar. The plates were incubated overnight at 37°C, and the MBCs were determined after 24 h.
Skin infection by the methicillin‑resistant Staphylococcus aureus strains in animals
All animal experiments were conducted according to the experimental practices and standards approved by the Animal Welfare and Research Ethics Committee of our Institutions. Twenty male mice aged 6-8 weeks and ranged in weight from 20 to 30 g were used. These mice were divided into four groups of five animals in each group. The group I was treated with Ag NPs ointment; group II was treated with allicin ointment; group III was treated with their combination; and group IV was the control (without Ag NPs and allicin). At first, the mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine, the hair on their neck was shaved and the skin disinfected with iodine. Then, a wound with length of 1 cm was generated, and 1.5 × 108 CFU/mL bacteria were inoculated.[22] Within three days, the wound was completely infectious, and afterward, different treatments were applied according to the specified group. To investigate the status of the infected wounds, four days after treatments with various ointments, animals were euthanized, and samples were collected and cultured by sterile swab. The colony counts were performed, and the results of CFU/mL were reported. In all steps of this study, animals were maintenance at animal house with 21-25°C and humidity 44-50 and lighting for 12 h of light and 12 h dark period. Daily water was prepared from tap water were replaced and provided enough food. (Fed taken from Bhprvar Company, Iran).
Statistical analysis
The extracts were prepared in triplicate (biological replicates) for chemical characterization and antibacterial assays, which were carried out in triplicate (technical replicates). Data were subjected to analysis of variance following a completely random design to determine the least significant difference at P < 0.01 using SPSS v. 11.5 (IBM SPSS, New York, USA).
Results
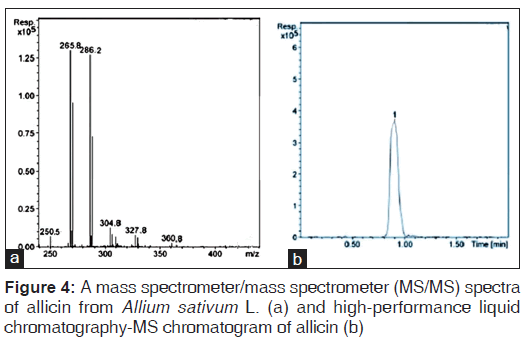
Analytical methods detecting allicin in ultraviolet at 220 nm[23,24] may present interferences because of low selectivity. The sulphur containing ingredients have the capacity to form adduct complexes with some common metals. The complex possesses an electric charge, and it can be analyzed by mass spectrometry with electrospray ionization. In order to obtain selectivity in a quantitative determination of allicin by HPLC‑MS, we utilized the adduct complex formed by allicin and the silver ion.[25] The peak of allicin was observed at RT = 0.8 min [Figure 4], and 135.5 mg/mL of allicin were measured.
The results of in vitro assays (microdilution test) showed that MIC of allicin and Ag NPs on MRSA strains were 2.2 (0.01) mg/mL, 5.6 (0.01) mg/mL, respectively, whereas MBC of allicin and Ag NPs on MRSA strains were 3.1 (0.01) mg/mL and 7.5 (0.03) mg/mL, respectively. However, MIC and MBC of allicin and Ag NPs together on MRSA strains were 0.4 (0.02) mg/mL and 1.1 (0.01) mg/mL, respectively [Table 1]. The statistical analysis at P < 0.01 showed that combined treatment with allicin and Ag NPs possessed significant antimicrobial activity on MRSA strains. Furthermore, the allicin and Ag NPs had a synergistic effect on these bacteria.
| Treatment | MIC (mg/mL) | MBC (ppm) |
|---|---|---|
| Silver nanoparticles | 5.6 (0.01)a* | 7.5 (0.03)a |
| Allicin | 2.2 (0.01)b | 3.1 (0.01)b |
| Silver nanoparticles+allicin | 0.4 (0.02)c | 1.1 (0.01)c |
Table 1: Values of MIC and MBC of silver nanoparticles, allicin and their combination on MRSA strains
In the animal model, MRSA bacteria were counted on the 4th day after treatments, according to the CFU/mL reported in Table 2. Results showed that the number of bacteria in all groups was significantly different at P < 0.01 from the control group. Topical ointments with allicin and Ag NPs to wounds infected with MRSA strains were highly effective, even if the combination of nanoparticles and allicin showed a synergistic antimicrobial activity. In general, bacteria counted for control, Ag NPs, allicin and their combination were 377 × 108, 80 × 106, 43 × 105, and 0 CFU/mL, respectively.
| Group | CFU/mL |
|---|---|
| Control | 377×108 (0.01)a* |
| Silver nanoparticles | 80×106(0.09)b |
| Allicin | 43×105(0.01)c |
| Silver nanoparticles+allicin | 0 (0.00)d |
Table 2: Antibacterial activity, expressed as CFUs/mL, of topical treatments with silver nanoparticles, allicin and their combination on wounds infected with MRSA strains in mice
Discussion
The present work investigated the potential of using allicin and Ag NPs to overcome bacterial resistance to antibiotics. Garlic contains more than 30 sulfur compounds, responsible both for its characteristic aroma and medicinal properties.[26] Among these, allicin is considered a major bioactive component of garlic, showing a broad‑spectrum antimicrobial activity again Gram‑negative and Gram‑positive bacteria, including Staphylococcus species.[27] As previously introduced, S. aureus is a commensal microorganism and a major hospital‑acquired pathogen. Strains of this species are resistant to β‑lactams, particularly the MRSA strains, through a multi‑drug resistance (MDR) mechanism. Bacteria can acquire MDR deactivating antibiotics by β‑lactamases or extruding them by active efflux transporters.[28] Furthermore, antibacterial activity of allicin again MRSA strains has been demonstrated in vitro, both in mupirocin‑susceptible and mupirocin‑resistant strains. Mupirocin (pseudomonic acid produced by Pseudomonas fluorescent) is a standard product used to treat MRSA infections, even if resistance to this drug is increasing in methicillin‑resistant bacteria.[10]
Antimicrobial activity of Ag NPs can be due to the release of silver ions which interact with DNA bases and phosphate groups, thus hindering DNA replication. In addition, Ag NPs can exert antibiotic effects independently from ion release. They can attach to the cell membrane, thus altering the membrane potential and inhibiting respiration, a process which can generate reactive oxygen species and oxidative stress.[29] Ag NPs can also penetrate into the cell, where their bactericidal activity depends on nanosize and concentration.[14,30]
Silver nanoparticles have attracted much attention in the treatment of multi‑drug resistant bacterial infections and as topical application in wound healing.[31] Previous studies demonstrated that Ag NPs are effective bactericidal agents regardless of the drug‑resistance mechanisms of MRSA.[32] A size‑ and dose‑dependent bactericidal activity again MRSA and nanotoxicity to human epithelial cells of nanosilver was also reported.[32,33] Furthermore, antibiotic activity of Ag NPs has been shown on multi‑drug resistant bacteria different from MRSA, that is, multidrug‑resistant Pseudomonas aeruginosa, ampicillin‑resistant Escherichia coli and erythromycin‑resistant Streptococcus pyogenes.[34] Wound healing activity of Ag NPs was previously demonstrated in animal models. In particular, green synthesis of Ag NPs using two glycosaminoglycans, chondroitin sulfate and acharan sulfate, was recently reported. These new nanomaterials showed a significant wound healing activity after topical application in mice subjected to incision wounds.[35]
To the best of our knowledge, antimicrobial efficacy of the combined treatment with Ag NPs and allicin has not been earlier investigated, even if synergistic, additive and antagonistic effects were previously observed between these nanoparticles and commonly used antibiotics.[36] A possible mechanism to explain our results could be the blocking of quorum sensing, a process which controls microbial biofilm formation. Quorum sensing is a system of gene expression regulation in bacteria depending on the density of their population, and genes involved in biofilm production are activated at a critical population density, that is, with a cell‑density‑dependent stimulus. Microbial biofilm consists in a community of bacterial cells enclosed in a self‑produced matrix, mainly composed of polysaccharides, adherent to an inert or living surface. Therefore, chemoresistance of biofilm‑forming bacteria involves the matrix, which represents a physical and chemical barrier to antibiotics.[37,38] In these terms, in our experimental conditions, a combination of allicin and Ag NPs may exert an antibiofilm activity, targeting biofilm formation of bacteria, a process responsible for a large increase in resistance to antimicrobial agents. Interestingly, anti‑quorum sensing and antibiofilm activities of garlic extracts and Ag NPs, not used in combination, were previously reported on P. aeruginosa, Staphylococcus epidermidis, S. aureus and Escherichia coli.[39‑42]
Conclusion
We showed that the combined treatment with allicin and Ag NPs may represent a promising anti‑staphyloccocal strategy, improving the antimicrobial activity of both agents applied singly and overcoming the increasing antibiotic resistance of MRSA strains. However, further studies are needed to determine the exact mechanism (s) of action of the synergistic combination. In any case, this study will be a valuable report for researchers in this field. It clearly indicates a simple and effective way to inhibit bacterial in skin infection and provide an alternative strategy to find natural and effective antibiotics.
Acknowledgements
This research was self‑funded by Authors. We are also grateful to anonymous Reviewers for providing constructive criticism on the manuscript.
References
- Jarraud S, Mougel C, Thioulouse J, Lina G, Meugnier H, Forey F, et al. Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect Immun 2002;70:631‑41.
- Lepelletier D, Lucet JC. Controlling meticillin‑susceptible Staphylococcus aureus: Not simply meticillin‑resistant S. aureus revisited. J Hosp Infect 2013;84:13‑21.
- Brennan L, Lilliebridge RA, Cheng AC, Giffard PM, Currie BJ, Tong SY. Community‑associated meticillin‑resistant Staphylococcus aureus carriage in hospitalized patients intropical northern Australia. J Hosp Infect 2013;83:205‑11.
- Hoseini‑Alfatemi SM, Sharifi‑Rad J, Sharifi‑Rad M, Mohsenzadeh S, da Silva JA. Chemical composition, antioxidant activity and in vitro antibacterial activity of Achillea wilhelmsii C. Koch essential oil on methicillin‑susceptible andmethicillin‑resistant Staphylococcus aureus spp. 3 Biotech 2014; 1‑6.
- Abbasi‑Montazeri E, Khosravi AD, Feizabadi MM, Goodarzi H, Khoramrooz SS, Mirzaii M, et al. The prevalence of methicillin resistant Staphylococcus aureus (MRSA) isolates with high‑level mupirocin resistance from patients and personnel in a Burn center. Burns 2013;39:650‑4.
- Diep BA, Carleton HA, Chang RF, Sensabaugh GF, Perdreau‑Remington F. Roles of 34 virulence genes in the evolution of hospital‑ and community‑associated strains of methicillin‑resistant Staphylococcus aureus. J Infect Dis 2006;193:1495‑503.
- Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin‑induced diabetic rats.Phytomedicine 2006;13:624‑9.
- Meriga B, Mopuri R, MuraliKrishna T. Insecticidal, antimicrobial and antioxidant activities of bulb extracts of Allium sativum. Asian Pac J Trop Med 2012;5:391‑5.
- Dkhil MA, Abdel‑Baki AS, Wunderlich F, Sies H, Al‑Quraishy S. Anticoccidial and antiinflammatory activity of garlic in murine Eimeria papillata infections. Vet Parasitol 2011;175:66‑72.
- Cutler RR, Wilson P. Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin‑resistant Staphylococcus aureus. Br J Biomed Sci 2004;61:71‑4.
- Londhe VP, Gavasane AT, Nipate SS, Bandawane DD, Chaudhari PD. Role of garlic (Allium sativum) in various diseases: An overview. J Pharm Res Opin 2011;1:129‑34.
- Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect 1999;1:125‑9.
- Ellmore GS, Feldberg RS. Alliin lyase localization in bundle sheaths of garlic clove (Allium sativum). Am J Bat 1994;81:89‑94.
- Dhama K, Tiwari R, Chakraborty S, Saminathan M, Kumar A, Karthik K, et al. Evidence based antibacterial potentials of medicinal plants and herbs countering bacterial pathogens especially in the era of emerging drug resistance: An integrated update. Int J Pharmacol 2014;10:1‑43.
- Snowden R, Harrington H, Morrill K, Jeane L, Garrity J, Orian M, et al. A comparison of the anti‑Staphylococcus aureus activity of extracts from commonly used medicinal plants. J Altern Complement Med 2014;20:375‑82.
- Pan X, Bligh SW, Smith E. Quinolone alkaloids from Fructus Euodiae show activity against methicillin‑resistant Staphylococcus aureus. Phytother Res 2014;28:305‑7.
- Saeidi S, Ravan H, Sanadgol N, Khaleghi M, Bazi S, Shojaei P. Methicillin‑resistance Staphylococcus aureus in Southeast Iran: Herbal control and detection methods comparison. J Med Sci 2014;14:123‑29.
- Rai MK, Deshmukh SD, Ingle AP, Gade AK. Silver nanoparticles: The powerful nanoweapon against multidrug‑resistant bacteria. J Appl Microbiol 2012;112:841‑52.
- Vlasea L, Parvub M, Parvuc EA, Toiud A. Phytochemical analysis of Allium fistulosum L. and A. ursinum. Dig J Nanomater Biostructures 2013;8:457‑67.
- Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 1998;64:711‑3.
- Al‑Fatimi M, Wurster M, Schröder G, Lindequist U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J Ethnopharmacol 2007;111:657‑66.
- Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR.Photodynamic therapy for methicillin ‑ resistant Staphylococcus aureus infection in a mouse skin abrasionmodel. Lasers Surg Med 2010;42:38‑44.
- Arnault I, Christidès JP, Mandon N, Haffner T, Kahane R, Auger J. High‑performance ion‑pair chromatography method for simultaneous analysis of alliin, deoxyalliin, allicin and dipeptide precursors in garlic products using multiple mass spectrometry and UV detection. J Chromatogr A 2003;991:69‑75.
- Vlase L, Pârvu M, Toiu A, Pârvu AE, Codruta CS, Mihai P. Rapid and simple analysis of allicin in Alium Species by LCCISMS/MS. Studia UBB Chem 2010;5:297‑304.
- Cardellina JH. Review of garlic and other alliums. The lore and the science. J Nat Prod 2013;76:813.
- Gebreyohannes G, Gebreyohannes M. Medicinal values of garlic: A review. Int J Med Med Sci 2012;5:401‑8.
- Gibbons S. Anti‑staphylococcal plant natural products. Nat Prod Rep 2004;21:263‑77.
- Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology 2005;16:2346‑53.
- Ayala‑Núñez NV, Villegas HH, Turrent LD, Padilla CR. Silver nanoparticles toxicity and bactericidal effect against methicillin‑resistant Staphylococcus aureus: Nanoscale does matter. Nanobiotechnology 2009;5:2‑9.
- Maillard JY, Hartemann P. Silver as an antimicrobial: Facts and gaps in knowledge. Crit Rev Microbiol 2013;39:373‑83.
- Ayala‑Nunez NV, Villegas HH, Turrent LC, Padilla CR. Silver nanoparticles toxicity and bactericidal effect against methicillin resistant Staphylococcus aureus: Nanoscale does matter. Nanobiotechnology 2009;5:2‑9.
- Ansari MA, Khan HM, Khan AA, Malik A, Sultan A, Shahid M, et al. Evaluation of antibacterial activity of silver nanoparticles against MSSA and MRSA on isolates from skin infections. Biol Med 2011;3:141‑6.
- Lara HH, Ayala‑Núñez NV, Turrent LC, Padilla CR. Bactericidal effect of silver nanoparticles against multidrug‑resistant bacteria. World J Microb Biotechnol 2010;26:615‑21.
- Im AR, Kim JY, Kim HS, Cho S, Park Y, Kim YS. Wound healing and antibacterial activities of chondroitin sulfate‑ and acharan sulfate‑reduced silver nanoparticles. Nanotechnology 2013;24:395102.
- Jain J, Arora S, Rajwade JM, Omray P, Khandelwal S, Paknikar KM. Silver nanoparticles in therapeutics: Development of an antimicrobial gel formulation for topical use. Mol Pharm 2009;6:1388‑401.
- Brackman G, Cos P, Maes L, Nelis HJ, Coenye T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother 2011;55:2655‑61.
- Taraszkiewicz A, Fila G, Grinholc M, Nakonieczna J. Innovative strategies to overcome biofilm resistance. Biomed Res Int 2013;2013:150653.
- Bjarnsholt T, Jensen PØ, Rasmussen TB, Christophersen L, Calum H, Hentzer M, et al. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology 2005;151:3873‑80.
- Harjai K, Kumar R, Singh S. Garlic blocks quorum sensing and attenuates the virulence of Pseudomonas aeruginosa. FEMS Immunol Med Microbiol 2010;58:161‑8.
- Kalishwaralal K, BarathManiKanth S, Pandian SR, Deepak V, Gurunathan S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf B Biointerfaces 2010;79:340‑4.
- Mohanty S, Mishra S, Jena P, Jacob B, Sarkar B, Sonawane A. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch‑stabilized silver nanoparticles. Nanomedicine 2012;8:916‑24.
- Radzig MA, Nadtochenko VA, Koksharova OA, Kiwi J, Lipasova VA, Khmel IA. Antibacterial effects of silver nanoparticles on Gram‑negative bacteria: Influence on the growth and biofilms formation, mechanisms of action. Colloids Surf B Biointerfaces 2013;102:300‑6.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.