Assessment of Red Blood Cell Parameters and Peripheral Smear at Different Temperatures in Case of Cold Agglutination Disease
- *Corresponding Author:
- Dr. Vivek Gupta
Department of Pathology, UP Rural Institute of Medical Science and Research, Saifai, Etawah - 206 301, Uttar Pradesh, India.
E-mail: dr_vivek_gupta@yahoo.com
Abstract
Cold agglutination disease (CAD) is characterized by an auto‑antibody which is able to agglutinate red blood cells (RBCs) at temperatures lower than that of the body, and subsequently to activate the complement system responsible for lysis of RBCs. Patients show hemolytic anemia of varying degrees of severity, which arise or worsen upon exposure to low temperatures. We describe a case who presented with fever and symptoms of asthenia. His investigations yielded bizarre RBC parameters which led to suspicion of a rare CAD, which was confirmed on reviewing RBC parameters, peripheral smear and direct Coomb’s test at different temperatures. Hence, we suggest assessment of bizarre RBC parameters and peripheral smear can help in laboratory testing and diagnosis of CAD. It should also not pose embarrassment in laboratory testing to the pathologist for making an early and accurate diagnosis, thus emphasizing the need for an early treatment of CAD.
Keywords
Cold agglutination disease, Peripheral smear, Red blood cell parameters
Introduction
Cold agglutinins were first described by Landsteiner in 1903.[1] Their pathological action against red blood cells (RBCs) (hemolytic anemia) and blood vessels (Raynaud’s syndrome) was described some years later by Clough and Iwai.[2,3] The association of cold hemagglutination with hemolysis was described in 1937 by Rosenthal and Corten.[4] In 1953, Schubothe coined the term: Cold agglutinin disease (CAD).[5]
CAD is characterized by an auto-antibody[6] usually of the immunoglobulin M (IgM) subclass, which binds to red cell antigens causing red cell agglutination at temperatures lower than that of the body. Subsequently, the IgM autoantibodies activate the complement system leading to both intravascular and extravascular lysis of RBCs. Intravascular hemolysis occurs due to direct complement mediated lysis while extravascular hemolysis is as a result of C3b coated red cell destruction by macrophages in the liver. Patients show hemolytic anemia of varying degrees of severity, as well as episodes of hemoglobinuria and acrocyanosis, which arise or worsen upon exposure to low temperatures.
The IgM antibodies resulting in cold agglutination hemolytic disease can be polyclonal or monoclonal. Polyclonal or post-infectious cold agglutinins are most notably seen with viral infections such as mycoplasma pneumonia, influenza A and B, infectious mononucleosis, and human immunodeficiency virus. Disorders associated with the production of a monoclonal IgM protein included splenic marginal zone lymphoma, small lymphocytic lymphoma, and lymphoplasmacytic lymphoma.
Case Report
The case we present here is about an 18 year-old man who presented to our hospital with fever and symptoms of asthenia (weakness). On examination, he had pallor and mild fever. Chest examination did not reveal any abnormality. No hepatosplenomegaly was present. No neuropathy was seen. There was no history of exposure to extremes of temperature and patient had no past history of any similar episode or any illness. No similar history was present in his relatives. A routine complete blood count (CBC) and peripheral smear examination were sent to our diagnostic laboratory.
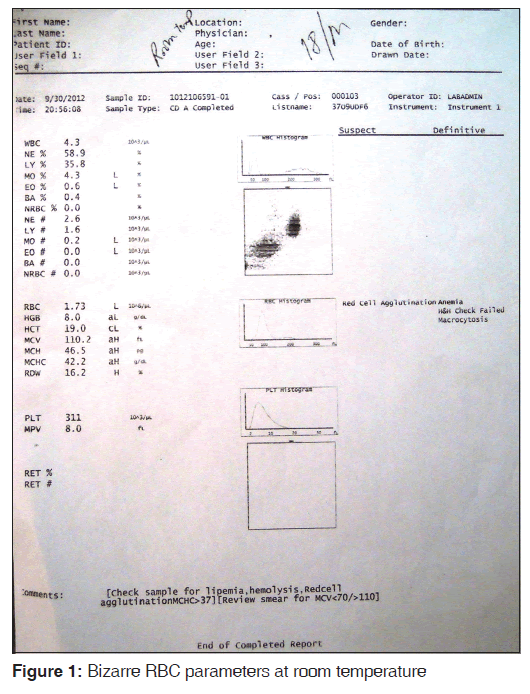
The sample was analyzed by Beckmen LH-750 Coulter. Findings were as follows: RBC 1.73 million, hemoglobin (Hb) 8.0 g/dl, hematocrit (Hct) 0.19 L/L, mean corpuscular volume (MCV) 110 fL, mean corpuscular hemoglobin (MCH) 46.5 pg, MCH concentration (MCHC) 42.2 g/dl, red cell distribution width 16.2. White blood cell 4.3 × 109/L differential leukocyte count: Neutrophils 58.9%, lymphocytes 35.8%, monocytes 4.3%, eosinophils 0.6%, basophils 0.4%, platelets 311 × 109/L. The CBC showed a suspected flag of red cell agglutination and a definitive message of anemia and macrocytosis and Hb and Hct check failed [Figure 1].
Sample had no evidence of hemolysis or lipemia. Peripheral smear examination was done which showed the presence of few red cell clumps, along with macrocytic normochromic cells.
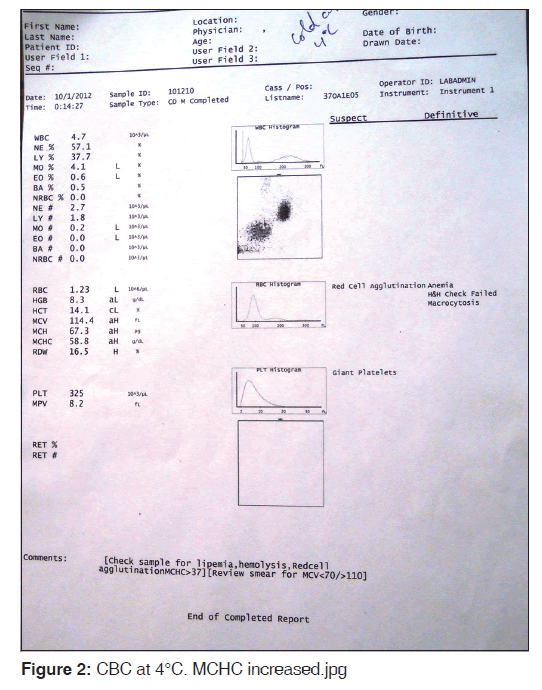
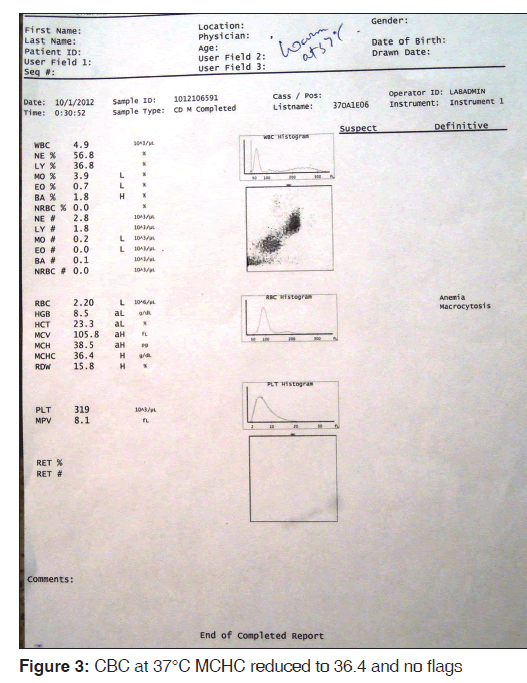
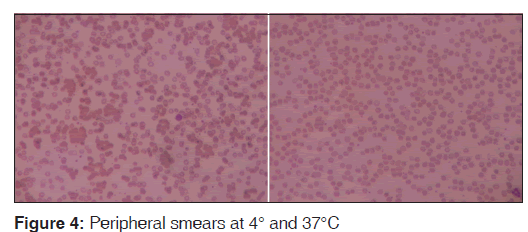
Sample was divided into two parts: the first one kept at 4°C and the other at 37°C for 15 min. After 15 min, the CBC was done again and the smears reviewed for each. The findings were compared. MCHC increased to 58.8 g/dl at 4°C [Figure 2] and reduced to 36.4 at 37°C [Figure 3]. Peripheral smear showed marked clumping of RBCs from the smear prepared from 4°C and there was no clumping observed at 37°C [Figure 4]. A comparative finding at different temperatures is shown in Table 1.
| Parameters | Findings at 4°C | Findings at 37°C | Findings at room temp |
|---|---|---|---|
| Hemoglobin | 8.3 | 8.5 | 8.0 |
| RBC (x106/µl) | 1.23 | 2.20 | 1.73 |
| Hematocrit (%) | 14.1 | 23.3 | 19.0 |
| MCV (fL) | 114.4 | 105.8 | 110.2 |
| MCH (pg) | 67.3 | 38.5 | 46.5 |
| MCHC (g/dl) | 58.8 | 36.4 | 42.2 |
| RDW (%) | 16.5 | 15.8 | 16.2 |
| WBC TLC (x103/µL) | 4.7 | 4.9 | 4.3 |
| DLC (%) | P‑57.1%, L‑37.7%, E‑0.6%, | P‑56.8%, L‑36.8%, E‑0.7%, | |
| M4.1%, B‑ 0.5% | M‑3.9%, B‑1.8% | ||
| Platelet count (x103/µL) | 325 | 319 | 311 |
| Peripheral smear | Most erythrocytes are agglutinated | Erythrocytes show normal | Erythrocytes are |
| findings | in variably large clumps | distribution and no clumps are noted | agglutinated in clumps |
TLC: Total leucocyte count, RBC: Red blood cell, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: MCH concentration, RDW: Red cell distribution width, WBC: White blood cell, DLC: Differential Leucocyte Count
Table 1: Red blood cell parameters and peripheral smear comparison of findings at different temperatures
The provisional diagnosis of CAD was made and a direct antiglobulin test with combined IgG and anti-C3 was done at 4°C and 37°C. The result was positive at 4°C and negative at 37°C.
Patient refused further intervention or workup, was discharged against medical advice and was lost to follow-up.
Discussion
The incidence of autoimmune hemolytic anemia (AIHA) is estimated to be approximately 1:100,000 in adults.[7] Among these, warm-reactive antibodies are responsible for 87% of the cases and cold reactive antibodies are responsible for 13% of the cases.[4] Occurrence of mixed type of AIHA is rare with <5% of all cases of AIHA reported.[8] In contrast, the south Indian data shows that cold AIHA is rare and that it contributes to only 1% of the total cases of AIHA.[9]
The paradox in CAD is the exacerbations that occur during febrile illnesses, which were seen in our patient. The most plausible explanation for these observations is that a majority of the CAD patients have low levels of C3 and C4 during their steady states, due to continuous complement consumption. During acute phase reactions, the C3 and C4 levels increase due to their enhanced production, resulting in the exacerbation of hemolysis.[10]
It has also been reported by Kakkar et al. that hematological parameters MCV, MCH and MCHC show spurious values in CAD[11] but the parameters are corrected at incubation of sample at 37°C. The similar findings are observed in our case. Incubation of the sample at 37°C in most cases restores the parameters to normal as the agglutination is reversed. The antibodies lose their potency at this temperature because they dissociate from the red cells.[11] However, falsely elevated MCV, MCH, MCHC and low RBC due to agglutination may occur in cold parts of automated counters, hence careful evaluation of peripheral smear should be done.
The polyclonal or post-infectious CAD usually occurs at a younger age than monoclonal, an outline of investigations should be done in such patients to find out the primary cause of CAD and these investigations would include reticulocyte count, serum lactate dehydrogenase, serum bilirubin, urine analysis (hemoglobin), urine immunoelectrohoresis, and chest radiograph. As our patient refused further investigations and left unceremoniously the primary cause of CAD could not be identified.
The treatment modality which can be used to suppress the production of these antibodies is immunosuppressive therapy with cyclophosphamide or chlorambucil. Rituximab, a monoclonal CD 20 antibody, and fludaribine, a purine analog, have proven to be of therapeutic use.[10] Plasmapheresis can be used as a temporary measure for the removal of the autoantibodies. Blood transfusions can be useful to tide over acute illnesses. Since the major hemolysis occurs in the liver, splenectomy is of minimal use.[10]
In the present case, an 18-year male presented with fever and pallor. The hemogram showed erratic values of the RBC count and the blood indices. The peripheral blood smear showed the spontaneous agglutination of RBCs. This problem was encountered while the patient was asked to get CBC and peripheral smear examination.
CAD is not an indolent disease in terms of the major clinical symptoms and the quality of life.[10] The problems that occur during the laboratory testing of CAD should not pose embarrassment to the pathologist to make an early and accurate diagnosis. The recent treatment modalities which consists of rituximab and fludaribine have shown good results, thus emphasizing the need for an early diagnosis of CAD.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Landsteiner K. Über beziehungen zwischen dem blutserum und den korperzeller. Munch Med Worchenschr 1903;50:1812.
- Clough MC, Richter IM. A study of an autoagglutinin occuring in a human serum. Johns Hopkins Hosp Bull 1918;29:86.
- Iwai S, Mei-Sai N. Etiology of Raynaud’s disease. Jpn Med World 1926;6:345.
- Rosenthal F, Corten M. Über das Phänomen der Autohämagglutination und über die Eigenscaften der ältehämagglutinine. Folia Haematol (Leipzig) 1937;58:64-90.
- Schubothe H. The cold hemagglutinin disease. Semin Hematol 1966;3:27-47.
- Petz LD, Garraty G. Acquired Immune Hemolytic Anemias. New York: Churchill Livingstone; 1980. p. 63-76.
- Powers A, Silberstein LE. Autoimmune haemolytic anaemia. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Silberstein LE, McGlave P, et al., editors. Hematology: Basic Principles and Practice. 5th ed. Philadelphia: Churchill Livingstone; 2008. p. 645-67.
- Sokol RJ, Hewitt S, Stamps BK. Autoimmune haemolysis: An 18-year study of 865 cases referred to a regional transfusion centre. Br Med J (Clin Res Ed) 1981;282:2023-7.
- Alwar V, Shanthala DA, Sitalakshmi S, Karuna RK. Clinical patterns and hematological spectrum in autoimmune hemolytic anemia. J Lab Physicians 2010;2:17-20.
- Berentsen S, Beiske K, Tjønnfjord GE. Primary chronic cold agglutinin disease: An update on pathogenesis, clinical features and therapy. Hematology 2007;12:361-70.
- Kakkar N, Makkar M. Red cell cytograms generated by an ADVIA 120 automated hematology analyzer: Characteristic patterns in common hematological conditions. Lab Med 2009;40:545-9.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.