Association between Periodontal Status, Oral Hygiene Status and Tooth Wear among Adult Male Population in Benin City, Nigeria
- *Corresponding Author:
- Dr. CC Azodo
Department of Periodontics, New Dental Complex, University of Benin Teaching Hospital, P. M. B. 1111 Ugbowo, Benin City, Edo State, Nigeria
E-mail: clementazodo@yahoo.com
Abstract
Background: The association between periodontal status, oral hygiene status and severity of tooth wear lesion varies from community to community and also from occupation to occupation. Aim: To determine the association between periodontal status, oral hygiene status and tooth wear among the adult male population in Benin City, Nigeria. Subjects and Methods: This study was conducted among 65 male drivers, aged 25-64 years, with a mean age of 48.6 (9.1) years in an organized private motor park in Benin City between November 2011 and January 2012. The data collected through interview and clinical oral examination were age, educational status, driving experience, oral hygiene status, periodontal status and severity of tooth wear. Results: In this study, 13.8% (9/65) and 1.5% (1/65) of the participants had 4-5 mm and ≥6 mm periodontal pockets, respectively. A total of 15.4% (10/65) of the participants had poor oral hygiene status and 58.5% (38/65) of the participants had tooth wear lesion, with 15.8% (9/65) being severe tooth wear lesions (score 3). Participants with poorer oral hygiene and more severe tooth wear lesions significantly exhibited poorer periodontal status. There also existed a significant association between oral hygiene status and the severity of tooth wear lesion among the participants in this study. Conclusion: Data from this study revealed a statistically significant association between periodontal status, oral hygiene status and severity of tooth wear lesion among the participants.
Keywords
Africa, Asia, Male population, Oral hygiene
Introduction
According to the community index of treatment needs (CPITN) developed by Ainamo, et al., (1982),[1] healthy periodontium means no gingival bleeding on probing and absence of calculus and periodontal pockets. Assessment of periodontal status by scoring gingival bleeding on probing, presence of calculus and periodontal pockets using CPITN indicates that it may be related to oral hygiene. Oral hygiene is documented as one of the determinants, most significantly associated with the variation in CPITN scores.[2]
Oral hygiene is the level of oral cleanliness of an individual, and is assessed based on the accumulation of soft and hard deposits on the surfaces of teeth, which are the etiological factors of periodontal diseases. The simplified oral hygiene index developed by Greene and Vermillion (1964) [3] is a simple, standard and acceptable method of assessing oral hygiene. Assessment of oral hygiene of individuals reasonably reflects their gingival and periodontal health, as the soft and hard deposits on the tooth are implicated in gingivitis, periodontitis, dental caries and halitosis. The newer studies linking periodontal disease with systemic problem have necessitated the need to assess oral hygiene of various categories of individuals in an attempt to provide preventive and interventional oral healthcare in order to halt the linked systemic health problems and, ultimately, improve the quality of life.
The maintenance of optimal oral hygiene is dependent on the efficacy of oral self-care, which includes the use of tooth brushes, chewing stick, dental floss and other interdental aids.
However, the improper use of these oral self-care methods could cause tooth abrasion, which is a form of tooth wear. The dietary factors like excessive consumption of hard diet and carbonated drinks and citrus fruits also result in attrition and erosion, respectively. Other factors found to correlate significantly with increased tooth (occlusal) wear are bruxism, pen and nailbiting, use of indigenous chewing-stick and high intake of fruit juices.[4] Tooth wear, which includes attrition, abrasion, abfraction and erosion, manifesting with dental, periodontal and joint consequences, is defined as the loss of dental hard tissue by a chemical or mechanical process that does not involve bacteria.[5]
The periodontal manifestation of tooth wear, like occlusal trauma, occurs due to the reduced ability of teeth to withstand the normal forces of mastication due to loss of tooth structure in tooth wear. The supervening plaque on occlusal trauma leads to accelerated periodontal breakdown and periodontitis. The tool for assessing the severity of tooth wear lesion, irrespective of type, is Smith and Knight tooth wear index (1984),[6] which measures and monitors tooth wear on all the four visible surfaces; buccal, cervical, lingual and occlusal–incisal (B, C, L, O, I) of all teeth present. It is simple to use clinically through intra-oral examination or from models and photographs, and has acceptable epidemiological inter- and intra-reproducibility.[7] The likelihood of having worse periodontal status among different occupation groups in reference to the professionals was highest for drivers justifying their selection for this study.[8]
The objective of the study was to determine the association between periodontal status, oral hygiene status and tooth wear among the adult male population in Benin City, Edo State.
Subjects and Methods
This study was conducted among drivers in an organized private motor park in Benin City between November 2011 and January 2012. Data were collected through interview and clinical oral examination. The interview elicited information on age, educational status and driving experience of the participants. The clinical examination was conducted in a well-illuminated office approved by the management of the private motor park and temporarily prepared for the research. The sterilized mouth mirrors, caries explorers and community periodontal index of treatment need epidemiological probes (CPITN-E) were used for the clinical examination of the participants, and the examiners wore examination gloves and disposable face mask during the clinical examination to prevent cross infection. The clinical oral examination was conducted to assess the periodontal status using CPTIN, oral hygiene status using simplified oral hygiene index and severity of tooth wear using the Smith and Knight index. The clinical examination was done by two periodontologists whose inter-examiner reliability was 95% at the pre-testing stage. The intra-examiner reliability of the periodontologists was also 95% during the study.
The scoring for CPITN were followed as suggested by Ainamo, et al.[1] on the following selected 17, 16, 11, 26, 27, 37, 36, 31, 46, 47 as
Code 0: Healthy periodontium, Code 1: Bleeding on probing, Code 2: Calculus present, Code 3: 4-5 mm periodontal pocket and Code 4: ≥6 mm periodontal pocket.
The oral hygiene status of the participants was grouped as: Good; 0-1.2, fair; 1.3-3.0 and poor; 3.1-6.0.
The severity of tooth wear was scored from 0 to 4 as follows:
• Score 0 – no loss of enamel surface characteristics on buccal/lingual/occlusal/incisal (B/L/O/I) and no change in contour on cervical region (C).
• Score 1 – loss of enamel surface characteristics on B/L/O/I and minimal loss of contour on C.
• Score 2 – loss of enamel exposing the dentine for less than 1/3 of the surface on B/L/O/I and defect less than 1 mm deep on C.
• Score 3 – loss of enamel exposing the dentine for more than 1/3 of the surface on B/L/O/I and defect 1-2 mm deep on C.
• Score 4 – complete loss of enamel or pulp exposure on B/L/O/I and defect more than 2 mm deep on C.
Informed consent was obtained from the participants after being informed of the study objective. The participants were also assured of the confidentiality of their responses. The data analysis was done using statistical package for social sciences (SPSS) version 17.0. Test of significance was done using Fischer’s exact statistics and statistical significance was set at P < 0.05.
Results
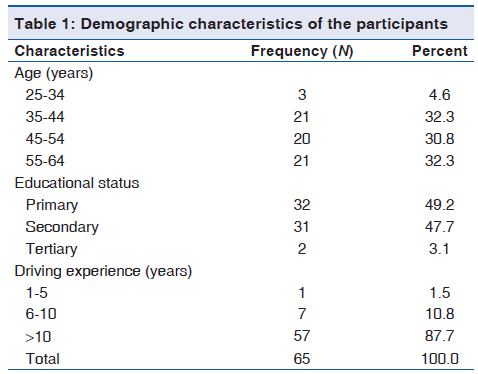
A total of 65 male drivers with a mean age of 48.6 (9.1) years in the private motor park participated in this study. Among the participants, (32.3%) 21/65 were aged 35-44 years, (49.2%) 32/65 had only primary school education and (87.7%) 57/65 have been professional drivers for more than 10 years [Table 1]. The prevalence of gingival bleeding on probing and calculus was (10.8%) 7/65 and (64.6%) 42/65, respectively. Among the participants, 9/65 (13.8%) had 4-5 mm periodontal pockets while 1/65 (1.5%) had ≥6 mm periodontal pockets. A total of 10/65 (15.4%) and 41/65 (63.1%) of the participants had poor and fair oral hygiene status, respectively. More than half (58.5%) of the participants had tooth wear lesion, with nine (15.8%) being severe tooth wear lesions (score 3). Educational attainment and driving experience were significantly associated with oral hygiene status, while only educational attainment was significantly associated was periodontal status. Demographic characteristics were not significantly associated with the severity of tooth wear lesion [Table 2].
| Variable | Total n (%) | Age (years) | Educational attainment | Driving experience | ||||
|---|---|---|---|---|---|---|---|---|
| 25-44 n (%) | 45-64 n (%) | Primary n (%) | Post-primary n (%) | ≤10 n (%)>10 n (%) | ||||
| OHI status | ||||||||
| Good | 14 | (21.5) | 9 (37.5) | 5 (12.2) | 3(9.4) | 11(33.3) | 4 (50.0) | 10(17.5) |
| Fair | 41 | (63.1) | 13(54.2) | 28(68.3) | 22(68.8) | 19(57.6) | 2 (25.0) | 39(68.4) |
| Poor | 10 | (15.4) | 2(8.3) | 8 (19.5) | 7 (21.9) | 3(9.1) | 2 (25.0) | 8 (14.0) |
| Fischer’s P | 0.05 | 0.04 | 0.04 | |||||
| CPITN score | ||||||||
| 0 | 6 | (9.2) | 3 (12.5) | 3(7.3) | 0(0.0) | 6 (18.2) | 1 (12.5) | 5(8.8) |
| 1 | 7 (10.8) | 4 (16.7) | 3(7.3) | 2(6.2) | 5 (15.2) | 2 (25.0) | 5(8.8) | |
| 2 | 42 | (64.6) | 14(58.3) | 28(68.3) | 23(71.9) | 19(57.6) | 4 (50.0) | 38(66.7) |
| 3 | 9 (13.8) | 3 (12.5) | 6 (14.6) | 6 (18.8) | 3(9.1) | 1 (12.5) | 8 (14.0) | |
| 4 | 1 | (1.5) | 0(0.0) | 1(2.4) | 1(3.1) | 0(0.0) | 0 (0.0) | 1(1.8) |
| Fischer’s P | 0.67 | 0.03 | 0.47 | |||||
| Tooth wear score | ||||||||
| 0 | 27 | (41.5) | 14(58.3) | 13(31.7) | 8 (25.0) | 19(57.6) | 4 (50.0) | 23(40.4) |
| 1 | 22 | (33.8) | 7 (29.2) | 15(36.6) | 13(40.6) | 9 (27.3) | 2 (25.0) | 20(35.1) |
| 2 | 10 | (15.4) | 1(4.2) | 9 (22.0) | 7 (21.9) | 3(9.1) | 1 (12.5) | 9 (15.8) |
| 3 | 6 | (9.2) | 2(8.3) | 4(9.8) | 4 (12.5) | 2(6.1) | 1 (12.5) | 5(8.8) |
| P value | 0.11 | 0.06 | 0.89 | |||||
CPITN: Community periodontal index of treatment need, OHI: Oral hygiene index
Table 2: Association between demographic characteristics, oral hygiene status, periodontal status assessed with CPITN and severity of tooth wears lesion among the participants
Older participants had higher debris, calculus and oral hygiene score, but age was significantly associated with only debris and oral hygiene score. Participants with lower educational attainment had significantly higher debris, calculus and oral hygiene score, while older participants in terms of driving experience had non-significant higher debris, calculus and oral hygiene scores. Participants with poorer periodontal status assessed with CPITN had significantly higher debris, calculus and oral hygiene score. The severity of tooth wear lesion was only significantly associated with calculus score [Table 3].
| Variables | Debris scoreMean (SD) | Calculus scoreMean (SD) | OHI scoreMean (SD) |
|---|---|---|---|
| Age (years) | |||
| 25-44 | 0.84 (0.60) | 0.70 (0.56) | 1.54 (1.08) |
| 45-64 | 1.41 (0.58) | 0.97 (0.58) | 2.38 (1.06) |
| t, P value | 3.79, <0.01 | 1.83, 0.07 | 3.09, <0.01 |
| Educational | |||
| attainment | |||
| Primary | 1.41 (0.61) | 1.07 (0.58) | 2.48 (1.04) |
| Post-primary | 0.99 (0.62) | 0.69 (0.54) | 1.68 (1.09) |
| t, P value | 2.71, <0.01 | 2.75, <0.01 | 3.00, <0.01 |
| Driving experience | |||
| (years) | |||
| ≤10 | 0.79 (0.81) | 0.77 (0.82) | 1.56 (1.59) |
| >10 | 1.25 (0.61) | 0.89 (0.56) | 2.14 (1.05) |
| t, P value | 1.93, 0.06 | 0.52, 0.60 | 1.36, 0.18 |
| CPITN score | |||
| 0 | 0.50 (0.41) | 0.00 (0.00) | 0.50 (0.41) |
| 1 | 0.95 (0.71) | 0.50 (0.48) | 1.45 (1.13) |
| 2 | 1.31 (0.61) | 0.97 (0.47) | 2.28 (0.99) |
| 3 | 1.22 (0.65) | 1.17 (0.67) | 2.39 (1.12) |
| 4 | 2.00 (0.00) | 2.00 (0.00) | 4.00 (0.00) |
| F, P value | 3.04, 0.02 | 8.50, <0.01 | 6.16, <0.01 |
| Tooth wear score | |||
| 0 | 1.09 (0.65) | 0.64 (0.54) | 1.73 (1.12) |
| 1 | 1.32 (0.68) | 0.99 (0.44) | 2.31 (0.99) |
| 2 | 1.35 (0.59) | 1.17 (0.74) | 2.52 (1.24) |
| 3 | 0.96 (0.64) | 1.01 (0.72) | 1.97 (1.32) |
| 4 | - | - | - |
| F, P value | 0.96, 0.42 | 2.97, 0.04 | 2.17, 0.17 |
| Total | 1.20 (0.65) | 0.87 (0.59) | 2.07 (1.13) |
CPITN: Community periodontal index of treatment need, OHI: Oral hygiene index
Table 3: Association between demographic characteristics, oral hygiene status, periodontal status assessed with CPITN and severity of tooth wear lesion among the participants
Oral hygiene was significantly associated with periodontal status, assessed using CPITN, as participants with poor oral hygiene were more likely to have poorer periodontal status [Table 4]. The severity of tooth wear lesion was also significantly associated with periodontal status, assessed with CPITN, as participants with more severe tooth wear lesions had poorer periodontal status [Table 5]. The oral hygiene status was significantly associated with the severity of tooth wear lesion as participants with poor oral hygiene status had more severe tooth wear lesions [Table 6].
| Code | OHI-S | Totaln (%) | Fischer’s P | ||
|---|---|---|---|---|---|
| Goodn (%) | Fairn (%) | Poorn (%) | |||
| 0 | 5 (35.7) | 1(2.4) | 0 (0.0) | 6(9.2) | <0.01 |
| 1 | 3 (21.5) | 4(9.8) | 0 (0.0) | 7 (10.8) | |
| 2 | 5 (35.7) | 30(73.2) | 7 (70.0) | 42(64.6) | |
| 3 | 1 (7.1) | 6 (14.6) | 2 (20.0) | 9 (13.9) | |
| 4 | 0 (0.0) | 0(0.0) | 1 (10.0) | 1(1.5) | |
| Total | 14 (100.0) | 41 (100.0) | 10 (100.0) | 65 (100.0) | |
CPITN: Community periodontal index of treatment need, OHI: Oral hygiene index
Table 4: Association between periodontal status assessed with CPITN and oral hygiene status among the participants
| Code | Tooth wear | Totaln (%) | Fischer’s P | |||||
|---|---|---|---|---|---|---|---|---|
| Score 0n (%) | Score 1n (%) | Score 2n (%) | Score 3n (%) | |||||
| 0 | 5(18.5) | 0(0.0) | 1(10.0) | 0 (0.0) | 6(9.2) | 0.01 | ||
| 1 | 5(18.5) | 1(4.6) | 0 (0.0) | 1 (16.7) | 7 (10.8) | |||
| 2 | 17(63.0) | 16(72.7) | 5(50.0) | 4 (66.6) | 42(64.6) | |||
| 3 | 0(0.0) | 5(22.7) | 3(30.0) | 1 (16.7) | 9 (13.9) | |||
| 4 | 0(0.0) | 0(0.0) | 1(10.0) | 0 (0.0) | 1(1.5) | |||
| Total | 27(100.0) | 22(100.0) | 10(100.0) | 6 (100.0) | 65 (100.0) | |||
CPITN: Community periodontal index of treatment need
Table 5: Association between periodontal status assessed with CPITN and tooth wear severity among the participants
| Toothwear | OHI-S | Totaln (%) | Fischer’s P | ||
|---|---|---|---|---|---|
| Goodn (%) | Fairn (%) | Poorn (%) | |||
| Score 0 | 9(64.3) | 16(39.0) | 2(20.0) | 27(41.5) | <0.01 |
| Score 1 | 3(21.4) | 15(36.6) | 4(40.0) | 22(33.8) | |
| Score 2 | 1 (7.1) | 6(14.6) | 3(30.0) | 10(15.4) | |
| Score 3 | 1 (7.1) | 4(9.8) | 1(10.0) | 6(9.2) | |
| Total | 14(100.0) | 41(100.0) | 10(100.0) | 65 (100.0) | |
OHI: Oral hygiene index
Table 6: Association between oral hygiene status and tooth wear severity among the participants
Discussion
In this study, 21.5% had good oral hygiene, which is comparable to the 18.0% documented in professional truck drivers in Mexico.[9] This may indicate that good oral hygiene is not common among professional drivers irrespective of geographical location and race. A total of 15.4% and 63.1% of the participants had poor and fair oral hygiene status, respectively, which indicated a low level of oral cleanliness among the participants. However, this is worse than the reported oral hygiene status among juvenile detainees in Udaipur city, Rajasthan, India,[10] and better than the oral hygiene status reported among Jain monks in Udaipur, India.[11] The avoidance of tooth cleaning, especially during fasting, among the monks may be the possible explanation.[11] The possible explanation for the poor oral hygiene status reported in this study is in three-folds. Firstly, the entire participants in this study were males who are known to exhibit suboptimal oral hygiene practices, poorer oral health knowledge, negative attitude to oral health and attach less importance to oral and general health.[12-14] Secondly, the constant mobility of these categories of individuals may affect their compliance with regular oral hygiene practices. Thirdly, low educational attainment adversely affects different forms of hygiene, namely bodily, oral and environmental, as poorer oral hygiene, higher plaque and calculus scores were evident among lower educationally attained participants in comparisonIn this study, 21.5% had good oral hygiene, which is comparable to the 18.0% documented in professional truck drivers in Mexico.[9] This may indicate that good oral hygiene is not common among professional drivers irrespective of geographical location and race. A total of 15.4% and 63.1% of the participants had poor and fair oral hygiene status, respectively, which indicated a low level of oral cleanliness among the participants. However, this is worse than the reported oral hygiene status among juvenile detainees in Udaipur city, Rajasthan, India,[10] and better than the oral hygiene status reported among Jain monks in Udaipur, India.[11] The avoidance of tooth cleaning, especially during fasting, among the monks may be the possible explanation.[11] The possible explanation for the poor oral hygiene status reported in this study is in three-folds. Firstly, the entire participants in this study were males who are known to exhibit suboptimal oral hygiene practices, poorer oral health knowledge, negative attitude to oral health and attach less importance to oral and general health.[12-14] Secondly, the constant mobility of these categories of individuals may affect their compliance with regular oral hygiene practices. Thirdly, low educational attainment adversely affects different forms of hygiene, namely bodily, oral and environmental, as poorer oral hygiene, higher plaque and calculus scores were evident among lower educationally attained participants in comparison with higher educational attainment participants. In this study, the prevalence of healthy periodontium (code 0) was 9.2%, signifying high level of periodontal diseases among the participants. This is lower than 10.7% among adult patients attending public health centers in the city of Recife, Brazil [15] and 12.5% rural in Ninevah Governorate,[16] but comparable to the 9.3% documented among factory workers in Romania. The stress dominating this profession in consonance with factory worker with its deleterious effect on the periodontium is an adduced explanation.[17,18]
The predominant prevalence of calculus (code 2) reported in this study has also been reported in studies conducted among Nigerian factory workers in Lagos State by Savage, et al.,[19] pensioners in Benin City by Okeigbemen, et al.,[20] rural populace in Ninevah Governorate[16] and adult patients attending public health centers in the city of Recife, Brazil (61.8%).[15] The low level of oral cleanliness seen among the participants in this study is a possible explanation as the recorded plaque, calculus and oral hygiene score were 1.20 (0.65), 0.87 (0.59) and 2.07 (1.13), respectively.
Among the participants in this study, 13.9% had 4-5 mm periodontal pockets (code 3), while 1.5% had ± 6 mm periodontal pockets (code 4). These findings are comparable to 14.1% code 3 and 1.3% code 4 reported in the urban–rural populace in Benin City by Akhionbare, et al.,[21] but lower than the 32.7% code 3 and 17.8% code 4 reported among factory workers in Romania by Roman, et al.[22] The little evidence of damage to the periodontal tissues despite the high prevalence of gingival bleeding on probing and calculus among participants has been attributed to the fibrous nature of diet among Nigerians in a previous study,[23] which may help reduce the periodontopathic bacteria population.
The pattern of periodontal status obtained in this study is comparable to the findings among adult patients attending public health centers in the city of Recife, Brazil, which is as follows: code 0 10.7%, code 1 10.3%, code 2 61.8%, code 3 15.2% and code 4 2.0%,[12] but better than the findings among pensioners in Benin City: 38.8% for code 3 and 6.8% for code 4 [20] the and Taiwo, et al.,[24] findings among the elderly in Ibadan, South Western Nigeria: 21.6% for code 3 and 28.8% for code 4. The fact that periodontal conditions are worse in older individuals is the possible explanation.[15,25,26] The finding in this study was also better than previously reported periodontal status in the national survey in Nigeria.[27] The improvement of oral health education and practices among Nigerians over the years and restriction of this study to the urban setting are the possible explanations.
In this study, more than half (58.5%) of the participants had tooth wear lesion, of which 23.7% had severe tooth wear lesions (score 3). This signified that the etiologies of tooth wear lesions, like abnormal tooth cleaning techniques, consumption of hard diet and erosive drinks, although not assessed in this study, may be dominant among the participants. The age of the participants contributed to the tooth wear, because tooth wear is known to be age related. Other forms of tooth wear, like abrasion, may have occurred mainly from wrong tooth brushing methods, and the stress of the profession may have activated parafunctional habits, worsening attrition, as majority have been professional drivers for more than 10 years. Overall, the prevalence of tooth wear lesion was found to be more among older participants in terms of driving experience.
Oral hygiene was significantly associated with periodontal status assessed using CPITN, as participants with poor oral hygiene were more likely to have poorer periodontal status. Poor oral hygiene results in poor periodontal status through direct mechanisms like the release of exotoxins, endotoxins, proteolytic and hydrolytic enzymes and toxic metabolic products, and indirect mechanisms through hypersensitivity reactions, activation of antigen antibody reaction and complement system activation.[28] The significant association between periodontal status, plaque, calculus and oral hygiene scores in this study may be a contributory explanation.
The severity of tooth wear lesion was also significantly associated with periodontal status assessed with CPITN, as participants with more severe tooth wear lesions had poorer periodontal status. The loss of tooth substance from tooth wear compromises the ability to withstand the normal forces of mastication, leading to occlusal trauma and eventual periodontal tissue destruction.
The significant association between oral hygiene status and the severity of tooth wear lesion found in this study may be explained by the fact that participants without tooth wear in comparison with participants with tooth wear score 1 and 2 using the Smith and Knight index,[6] had a higher plaque, calculus and oral hygiene score. The poor oral hygiene may exert an additive adverse effect on the compromised periodontium, resulting in periodontitis.
Conclusion
Data from this study revealed a statistically significant association between periodontal status, oral hygiene status and severity of tooth wear lesion among the participants. A significant association between oral hygiene status and severity of tooth wear lesion was also noted among the participants in this study.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Ainamo J, Barmes D, Beagrie G, Cutress T, Martin J, Sardo-Infirri J. Development of the World Health Organization (WHO) community periodontal index of treatment needs (CPITN). Int Dent J 1982;32:281-91.
- Marques MD, Teixeira-Pinto A, da Costa-Pereira A, Eriksen HM. Prevalence and determinants of periodontal disease in Portuguese adults: Results from a multifactorial approach. Acta Odontol Scand 2000;58:201-6.
- Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc 1964;68:7-13.
- Johansson A, Fareed K, Omar R. Analysis of possible factors influencing the occurrence of occlusal tooth wear in a young Saudi population. Acta Odontol Scand 1991;49:139-45.
- Tomasik M. Analysis of etiological factors involved in noncarious cervical lesions. Ann Acad Med Stetin 2006;52:125-36.
- Smith BG, Knight JK. An index for measuring the wear of teeth. Br Dent J 1984;156:435-8.
- Bardsley PF. The evolution of tooth wear indices. Clin Oral Investig 2008;12:15-9.
- Morita I, Nakagaki H, Yoshii S, Tsuboi S, Hayashizaki J, Igo J, et al. Gradients in periodontal status in Japanese employed males. J Clin Periodontol 2007;34:952-6.
- Aguilar-Zinser V, Irigoyen ME, Rivera G, Maupomé G, Sánchez-Pérez L, Velázquez C. Cigarette smoking and dental caries among professional truck drivers in Mexico. Caries Res 2008;42:255-62.
- Agrawal A, Bhat N, Shetty S, Sharda A, Singh K, Chaudhary H. Oral hygiene and periodontal status among detainees in a juvenile detention center, India. Oral Health Prev Dent 2011;9:281-7.
- Jain M, Mathur A, Kumar S, Duraiswamy P, Kulkarni S. Oral hygiene and periodontal status among Terapanthi Svetambar Jain monks in India. Braz Oral Res 2009;23:370-6.
- Al-Ansari JM, Honkala S. Gender differences in oral health knowledge and behavior of the health science college students in Kuwait. J Allied Health 2007;36:41-6.
- Peker I, Alkurt MT. Oral health attitudes and behavior among a group of Turkish dental students. Eur J Dent 2009;3:24-31.
- Kateeb E. Gender-specific oral health attitudes and behaviour among dental students in Palestine. East Mediterr Health J 2010;16:329-33.
- Coelho Rde S, Gusmão ES, Jovino-Silveira RC, Caldas Ade F. Profile of periodontal conditions in a Brazilian adult population. Oral Health Prev Dent 2008;6:139-45.
- Khamrco TY. Assessment of periodontal disease using the CPITN index in a rural population in Ninevah, Iraq. East Mediterr Health J 1999;5:549-55.
- Genco RJ, Ho AW, Kopman J, Grossi SG, Dunford RG, Tedesco LA. Models to evaluate the role of stress in periodontal disease. Ann Periodontol 1998;3:288-302.
- Genco RJ, Ho AW, Grossi SG, Dunford RG, Tedesco LA. Relationship of stress, distress and inadequate coping behaviors to periodontal disease. J Periodontol 1999;70:711-23.
- Savage KO, Afolabi BM, John MO. Assessment of periodontal status of Nigerian factory workers in relation to cigarette smoking in Lagos. Nig Q J Hosp Med 1999;9:198-201.
- Okeigbemen SA, Jeboda SO, Umweni AA. A preliminary assessment of the periodontal status of elderly pensioners in Benin City, Nigeria. Gerodontology 2012;29:e1244-8.
- Akhionbare O, Ojehanon PI, Ufomata DO, Jeboda SO. Periodontal treatment needs of urban and rural populations in Edo State, Nigeria. Nig Dent J 2007;15:13-7.
- Roman A, Pop A. Community periodontal index and treatment needs values (CPITN) in a factory worker group in Cluj-Napoca, Romania. Int Dent J 1998;48:123-5.
- Kubota K, Watanabe H, Hollist NO, Ajayi-Obe SO, Ono Y, Ohnishi M, et al. Dental survey in Nigeria. Part 4. Prevalence and severity of periodontal diseases. Bull Tokyo Med Dent Univ 1988;35:11-7.
- Taiwo JO, Jeboda SO, Motayo TO, Obiechina AE. Periodontal health of the elderly people in South East local government area in Ibadan, Nigeria. Afr J Med Med Sci 2004;33:285-91.
- Garcia ML, Cutress TW. A national survey of periodontal treatment needs of adults in the Philippines. Community Dent Oral Epidemiol 1986;14:313-6.
- Krustrup U, Erik Petersen P. Periodontal conditions in 35-44 and 65-74-year-old adults in Denmark. Acta Odontol Scand 2006;64:65-73.
- Adegbembo AO, el-Nadeef MA. National survey of periodontal status and treatment need among Nigerians. Int Dent J 1995;45:197-203.
- Eley BM, Manson JD. Periodontics. 5th ed. Edinburgh (Scotland): Wright Publishing; 2010. p. 144-8.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.