Bone Cement Implantation Syndrome in Cemented Hip Arthroplasty: Hypothetization of a New Therapeutic Approach and Proposition of a Treatment Algorithm
Santa Casa de Misericórdia de Belo Horizonte, 30150-250, Belo Horizonte, MG, Brazil, Email: lucas5412@gmail.com
1 Hospital das ClÃnicas, Universidade Federal de Minas Gerais, 30130-100, Belo Horizonte, MG, Brazil
2 Hospital Municipal Miguel Couto, 22430-160, Rio de Janeiro, RJ, Brazil
Citation: Santos LEN, et al. Bone Cement Implantation Syndrome in Cemented Hip Arthroplasty: Hypothetization of a New Therapeutic Approach and Proposition of a Treatment Algorithm. Ann Med Health Sci Res. 2018;8:111-116
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Purpose: Management of bone cement implantation syndrome (BCIS) during or immediately after hip arthroplasty (HA) is difficult and often leads to mortality. We have reviewed the medical records of patients submitted to HA at our institution and propose a standard operating procedure (SOP) for the event of cardiogenic shock. Methods: SOP was based on the analysis of the medical records of patients who had been submitted to cemented (n = 250) or uncemented (n = 408) HA, three of whom died because of BCIS, and on the individual experiences of the surgical team, taking into consideration the pathophysiology of acute right ventricular failure. Results: The proposed SOP involves the use of extracorporeal membrane oxygenation (ECMO) as a complementary therapy to mechanical ventilation with the purpose of improving patient outcome and reducing the risk of death. Inclusion and exclusion criteria, along with recommendations for the use of ECMO, are presented. Conclusion: Although our hypothesis has yet to be fully validated, the proposed a SOP has been implemented in our hospital. Because of the rarity of BCIS, we hope that the SOP will be effectuated in other institutions to ascertain the effectiveness of ECMO in the treatment of BCIS.
Keywords
Bone cement; Hip replacement; Pulmonary embolism; Cardiogenic shock
Introduction
Cemented Total Hip Arthroplasty (THA) is a reconstructive technique developed by Charnley [1] in the 1960s in which the hip joint is replaced with a tribological prosthetic pair comprising a metal femoral head articulated to a polyethylene acetabular component. The components are anchored to the bone using polymethyl methacrylate-based (PMMA) cement, a material that has been shown to provide reliable anchorage. However, despite the widespread success of cemented THA, the procedure may give rise to various complications including aseptic loosening of the joint implant, osteolysis and bone cement implantation syndrome (BCIS). Although relatively rare, BCIS is a life-threatening complication characterized by systemic hypotension, pulmonary hypertension and decrease in blood oxygen saturation either at the time of cementation, during insertion of the prosthesis or after reduction of prosthetic components. [2,3] Parvizi et al. [4] reviewed the records of 29.431 individuals submitted to THA and reported that, of the 23 patients who had died during surgery, 11 had undergone cemented total THA and 12 cemented hemiarthroplasty, indicating a prevalence of cementing-induced irreversible cardiorespiratory disturbance of around 0.08%. BCIS also occurs in other surgical procedures that require cementation including knee arthroplasty and vertebroplasty. [5,6]
Our surgical team at the Hospital Felicio Rocho, Belo Horizonte, MG, Brazil performed 658 hip arthroplasties between January 2012 and July 2017, in which 250 procedures employed cemented techniques and 408 uncemented procedures. Three (1.2%) of the patients submitted to a cemented technique developed BCIS that resulted in death.
Faced with the gravity of BCIS and the difficulty of treating this condition, we reviewed the medical records of patients submitted to hip arthroplasty at our institution with the aim of proposing a resuscitation algorithm for the event of cardiogenic shock during or shortly after prosthesis insertion.
To the best of our knowledge, there is no standard operating procedure (SOP) to treat BCIS, hence we set out to devise a treatment algorithm based on the pathophysiology of acute right ventricular failure. We hypothesized that the use of extracorporeal membrane oxygenation (ECMO) as a complementary therapy to mechanical ventilation would improve the outcomes of patients presenting BCIS reducing the risk of mortality. This procedure is already used in cardiovascular emergencies that require mechanically assisted circulatory support and may be a rescue approach in selected cases. [7]
ECMO is a technique that uses catheters that through a machine and through pumps performs the function of the heart and or the lungs. ECMO removes blood out of the body, oxygenating it directly and again circulating it in the body of the individual. ECMO can be considered rescue therapy for patients with refractory hypoxemia, since, during its use, lung work becomes minimal. Typical cardiac indications include low refractory cardiac output and hypotension (systolic blood pressure <90 mmHg), despite adequate intravascular volume, high dose inotropic and intra-aortic balloon. It is also used as rescue therapy in acute respiratory failure to gain time while improving lung disease is expected, providing oxygenation and removing CO2, or both, while the lungs recover. [8]
The proposed standard operating procedure (POP) for BCIS treatment adds ECMO to conventional ventilatory support for gas exchange with minimal pulmonary effort until recovery of this organ. ECMO allows a lower PEEP to be used in mechanical ventilation, which reduces the potential deleterious effects of mechanical ventilation-induced lung injury, which often occurs when it is instituted alone.
A decrease in carbon dioxide concentration at the end of expiration may be the first indication of clinically significant BCIS in the anesthetized patient and should alert the anesthesiologist, in addition to hypotension and decreased saturation. Early signs of BCIS in the awake patient are dyspnea and mental confusion.
Patients with severe respiratory failure who do not demonstrate improvement in blood gases or reduced mechanical ventilation parameters after 4 to 6 hours with rescue maneuvers should already be evaluated by the specialists of the ECMO team.
Methods
Study population
The study population comprised 658 patients who had been submitted to total or hemi hip arthroplasties at the Hospital Felicio Rocho. The distribution of the study population according to gender and type of surgical procedure is presented in Table 1. The bone cement (Simplex P Bone Cement; Stryker, Mahwah, NJ, USA) employed in the cementation procedures contained: methylmethacrylate-styrene copolymer, PMMA, barium sulfate (without Tobramycin). The three patients, two females and one male, who developed BCIS during surgery involving a cementation procedure, had an average age of 77.3 years.
| Procedure | Male (n) | Female (n) | Total (n) | BCISa (n) |
|---|---|---|---|---|
| Cemented total hip arthroplasty | 42 | 62 | 104 | 0 |
| Cemented bipolar hip hemiarthroplasty | 46 | 94 | 140 | 2 |
| Cemented monopolar hip hemiarthroplasty | 3 | 2 | 5 | 0 |
| Cemented endoprosthetic hip arthroplasty | 0 | 1 | 1 | 1 |
| Uncemented hip arthroplasty | 255 | 153 | 408 | 0 |
| Total | 346 | 312 | 658 | 3 |
Abone Cement Implantation Syndrome
Table 1: Distribution of the study population according to surgical procedures and gender.
Development of an SOP for patients presenting BCIS
A SOP was devised based on the analysis of medical records of the study population, the individual experiences of the surgical team and multidisciplinary discussion of the pathophysiology of acute right ventricular failure.
Inclusion and exclusion criteria for ECMO
Since the proposed SOP anticipates the use of ECMO, candidate patients must be assessed by an expert team regarding compliance with the criteria shown in Figure 1.
Mechanical complications of ECMO
Although mechanical complications during ECMO are relatively rare (~14% of all complications), the system should be inspected daily by a specialized technician. Problems often encountered are: (i) formation of blood clots in the ECMO circuit, mainly in the parts where blood flow is slow, despite administration of anticoagulant therapy to prevent thrombosis; (ii) failure of the oxygenator because of blood clot formation in the membrane (6% of complications). Alterations in gas exchange and reduced carbon dioxide transfer, as indicated by increased pre-membrane pressure, may indicate problems with the membrane and the need for replacement; (iii) entrainment of air in the ECMO circuit that may lead to embolism (4% of complications). This problem is generally caused by excessive negative pressure in the venous line, a complication that may averted by programming the system to decrease venous line pressure when excess levels are detected. Another cause of air embolism is excessive oxygen saturation after the blood passes through the oxygenator, and for this reason post-membrane PaO2 should be kept below 600 mmHg. If air bubbles are observed in the system, the pump should be stopped, the arterial line proximal to the patient should be clamped followed by the venous line and the air should be removed from the bridge between the two lines; (iv) rupture of the circuit (0.3% of complications) caused by either breakdown of circuit components (tubing, oxygenator, pump head) or increased pressure in the system. In such cases, ECMO must be withdrawn and emergency ventilation provided.
Results
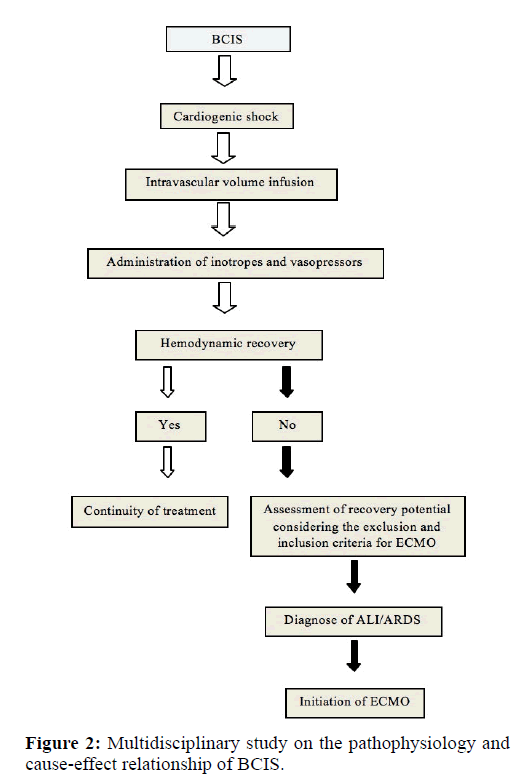
The proposed SOP for the event of cardiogenic shock during cemented hip arthroplasty is illustrated in Figure 2. Patients that do not respond to standard management procedures and persist with hypoxemia may improve their survival with temporary ECMO support. Should BCIS develop, the conventional management strategy includes mechanical ventilation with 100% O2, volume resuscitation with central venous pressure monitoring and use of α1 adrenergic agonists in the case of severe right ventricular dysfunction with hemodynamic instability. In such cases, the following recommendations are provided for cannulation, anticoagulant therapy, initiation of ECMO, daily monitoring, management of hypoxemia and mechanical complications and decannulation.
Prior to cannulation, consideration should be given to the results of coagulation tests including complete blood cell count, plasma fibrinogen test, activated partial thromboplastin time (APTT), international normalized ratio (INR) and activated clotting time (ACT). Cannulation should be performed percutaneously and in the presence of a cardiovascular surgeon, a clinical perfusionist, an equipment-trained intensive care unit (ICU) nurse, an ICU respiratory physiotherapist and an ECMO cardiologist. At the discretion of the medical team, echocardiography-guided cannulation at the bedside should be performed to facilitate correct positioning of the cannula.
Anticoagulant therapy with heparin, the antithrombotic agent most commonly used during ECMO, should commence at the time of cannulation. ACT is the preferential test for optimization of heparin dosage since it can be carried out at the bedside and the results are obtained immediately. Under basal conditions, ACT tests should be performed at least every 6 h. In addition, APTT should be performed as a complementary test at least twice a day. When the two tests diverge, a thromboelastogram must be performed to clarify the situation. Fibrinogen activity, D-dimer test and platelet count should be performed daily.
Anticoagulant therapy typically involves the intravenous administration of an initial 50-100 units/kg heparin bolus followed by continuous infusion. If ACT <300 s, the initial heparin infusion must be reduced to 7.5-20 units/kg. The typical maintenance dose of heparin is 17-20 units/kg/h and should not exceed 50 units/kg/h. Anticoagulant effects are generally acceptable when ACT ranges from 180-220 s or APTT ranges from 1.5-2.5 times the reference value.
After connection, the ECMO pump speed should be adjusted to achieve an initial blood flow of 500 mL/min until the entire system is filled with blood. Afterwards, blood flow can be increased to 2000 mL/min, while gas delivery should be set at 2000 mL/min. These parameters can then be modified in a 1:1 ratio until peripheral oxygen saturation is 90%. Thereafter, flow and sweep gas are adjusted in order to maintain PaO2 > 55 mmHg and pH ≥ 7.3. Mechanical ventilation should be adjusted to a positive end-expiratory pressure (PEEP) of 10 cm H2O, a fraction of inspired oxygen (FiO2) of 30% or the lowest value possible, an inspiratory pressure (IP) of 10 cm H2O and a respiratory rate (RR) of 10 breaths/min. The heat exchanger of the ECMO circuit should be monitored to maintain appropriate body temperature. Withdrawal of sedation and curare should be evaluated as soon as the hemodynamic conditions of the patient are stabilized.
Pre and post-membrane pressures, absence of blood clots in the system, auscultation of pump campanula and arterial pressure of the patient should be checked daily not only to optimize the ventilator parameters and the ECMO circuit, but also to evaluate the conditions of the circuit components. The patient should be submitted to laboratory tests daily in order to check that hematocrit remains, preferably, above 30% and platelets above 50,000/mm3, and the position of the cannula should be monitored diurnally. Transthoracic echocardiography should be performed if the cannula was originally inserted without such assistance, and repeated whenever there is clinical worsening or at the discretion of the medical team, in order to prevent potential complications such as pericardial effusion or ventricular dysfunction. Early tracheostomy within 72 hours after the beginning of ECMO should be considered if there is absence of sensorium or need of higher doses of sedatives to control psychomotor agitation.
If an ECMO patient presents hypoxemia with normal or near normal PCO2, the following strategies should be adopted in order to reestablish oxygen saturation to ≥ 85%, as recommended by Nunes et al. [9]: (i) optimize the extracorporeal membrane oxygenation blood flow in accordance with the cardiac output of the patient; (ii) identify recirculation problems and consider repositioning the cannula; (iii) optimize residual lung function; (iv) identify oxygenator dysfunction and consider replacement; and (v) optimize the extracorporeal membrane oxygenation blood flow by reducing the cardiac output of the patient through administration of antipyretics, sedatives or curare among other agents. Optimization of oxygen supply through blood transfusion should always be considered.
Conditions for weaning the patient off ECMO should be evaluated on a daily basis according to the following criteria: (i) under supportive pressure, the respirator data remains constant, and (ii) under controlled ventilation, the plateau pressure (Pplat) <25 cm H2O, tidal volume (TV) = 6 mL/kg, FiO2 = 60% and IP <15 cm H2O. Weaning should start by interrupting the gas flow and reducing the blood flow to 2000 mL/min, but the process should be discontinued if problems arise such as oxygen saturation <85%, respiratory rate (RR) > 35 breaths/min, use of accessory musculature, hypotension or onset of diaphoresis. However, if hemodynamic conditions are stable (as defined by PaO2 > 55 mmHg and pH > 7.3) and the patient breathes without effort, decannulation can proceed after 1 hour. Reversal of heparin effects with protamine sulfate is not mandatory, although heparin administration should be discontinued at least 2 hours before decannulation.
Discussion
BCIS is an inherent complication of cemented arthroplasty and is characterized by cardiogenic shock following cementation of the prosthetic components. We retrospectively identified three cases (1.2%) grade 3 of BCIS classification that resulting in death at our hospital within a period of five years. [10] In one case, the patient survived the first 24 h and remained in ICU for 13 days. In view of the obscure pathophysiology and the high lethality rate of BCIS, we sought to establish an SOP that could be applied to minimize the morbimortality of the syndrome.
It is known that BCIS is caused by the hemodynamic effects of embolization and the resulting increase in pulmonary artery pressure and, ultimately, right ventricular failure. Embolization is triggered by high intramedullary pressures between the bone and the cement used as filler for prosthesis insertion, which displace bone marrow, fat, air and other medullary debris into the pulmonary vascular compartments. The presence of emboli together with vasoactive substances increases pulmonary vascular pressure and resistance leading to rapid acute dilation of the right ventricle and dislocation of the interventricular septum towards the left ventricle within the constraints of the pericardial cavity. Such alterations reduce the left ventricular chamber and the amount of blood that normally fills this ventricle so that left ventricle cardiac output is reduced causing systemic arterial hypotension. The consequent reduction in coronary perfusion pressure and right coronary flow leads to hypoxia, ischemia and, ultimately, sudden cardiac failure. [11]
Although BCIS is a transient phenomenon, the effects may persist for more than 48 hours after surgery. Some studies have indicated that pulmonary arterial pressure normalizes within 24 hours after the onset of symptoms. [12] However, the capacity of the patient to tolerate cardiorespiratory changes depends on the cardiopulmonary reserve and number of emboli released into the pulmonary vascular system. [13] Advanced age, low cardiorespiratory reserve, right ventricular dysfunction, coronary disease, tumors and bone metastasis (bone hypervascularization) are considered risk factors for BCIS. [14]
Despite the low incidence of BCIS, evidence of perioperative fat and bone marrow embolism has been detected by transesophageal echocardiography (TEE) in 90-98% of patients submitted to cemented hip arthroplasty, although not all patients presented clinical repercussions. [14-17] TEE provides clear images of the heart so that the formation and size of emboli can be monitored and scored from grade 0 (absence of emboli) to 3 (emboli > 0.5 mm) during arthroplasty. [18] It seems that most patients compensate for the modest right ventricular afterload that follows embolization, suggesting that the determinant factor of cardiac arrest during/after cemented hip arthroplasty is not the number of emboli but the degree of hemodynamic overload and hypoxemia. [12] Thus, the echocardiographer should concentrate on the degree of dilation of the right ventricle and on the deviation of the interventricular septum rather than just the number and size of emboli. Moreover, some perioperative measures may help to prevent the development of BCIS such as copious pressure washing (especially in the femoral canal), vacuum suction of the femoral medullary canal during cementation, implantation of a more hardened cement and slow insertion of the femoral stem.
ECMO is a heart-lung bypass machine that oxygenates blood and removes carbon dioxide whilst maintaining patient lung work to a minimum. We have hypothesized that ECMO could be used as a complementary therapy since it reduces the potential deleterious effects of lung injury resulting from mechanical ventilation, thereby reducing the risk of mortality.
One of the most common forms of ECMO is the veno-venous technique (VV-ECMO) in which blood is drawn from a main vein via a cannula, passes through the ECMO oxygenator and returns via a cannula inserted in another main vein. VV-ECMO is especially beneficial to patients with severe respiratory failure that is refractory to conventional mechanical ventilation support. [19,20] Hence, it defends that, at the discretion of the medical team, patients with severe respiratory failure whose blood gases and mechanical ventilation parameters do not improve after 4-6 hours of rescue maneuvers should be evaluated by the ECMO team. Veno-arterial ECMO is an alternative form of oxygenation that can be used for patients with severe heart failure with or without respiratory failure, but in this case blood is drawn from a main vein, passes through the oxygenator and returns to a main artery (normally the aorta). Studies have shown that ECMO in either form substantially reduces mortality among patients with severe respiratory failure. [21-24]
Considering that current approaches do not prevent the catastrophic effects of embolization during cemented hip arthroplasty, we believe that BCIS patients could benefit from ECMO as long as the inclusion and exclusion criteria are satisfied [Figure 1]. Owing to the rarity of BCIS, our hypothesis has yet to be fully validated in our institution and this constitutes an important limitation of the study. In order to overcome this limitation and to provide evidence for or against our premise, we have undertaken a detailed multidisciplinary study on the pathophysiology and cause-effect relationship of BCIS and have proposed a SOP that has been implemented in our hospital [Figure 2].
Conclusion
It is known that ECMO allows the use of low PEEP parameters during mechanical ventilation, which reduces secondary damage to the lungs when compared to traditional mechanical ventilation with elevated PEEP. It would be ideal if other institutions participated in a randomized study with a greater number of cases of BCIS so that this protocol could be implanted and compared, through a robust statistical analysis, the treatment of BCIS with and without the use of ECMO.
We hope that this SOP will be effectuated in other institutions to ascertain the effectiveness of ECMO in the treatment of BCIS and to fill the gap in current management procedures.
However, we are fighting against a “strong enemy” and the aim of the present study was try to introduce a potential effective and alternative treatment option for BCIS, since the standard protocols need to be improved to increase survival rates. In addition, future studies are necessary to confirm the real impact of the ECMO in the survivorship of BCIS.
Ethical Statement
The project was submitted to and approved by the Research Ethics Committee of Hospital Felicio Rocho, Belo Horizonte, MG, Brazil (reference no. CEP 520/16).
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Charnley J. The long-term results of low-friction arthroplasty of the hip performed as a primary intervention. Bone Joint J. 1972;54:61-76.
- Rinecker H. New clinico-pathophysiological studies on the bone cement implantation syndrome. Arch Orthop Trauma Surg. 1980;97:263-274.
- Ereth MH, Weber JG, Abel MD, Lennon RL, Lewallen DG, Ilstrup DM, et al. Cemented versus noncemented total hip arthroplasty—embolism, hemodynamics, and intrapulmonary shunting. Mayo Clinic Proc. 1992;67:1066-1074.
- Parvizi J, Holiday AD, Ereth MH, Lewallen DG. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;369:39-48.
- Byrick RJ, Forbes D, Waddell JP. A monitored cardiovascular collapse during cemented total knee replacement. Anesthesiology. 1986;65:213-216.
- Chen HL, Wong CS, Ho ST, Chang FL, Hsu CH, Wu CT. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg. 2002;95:1060-1062.
- Bartlett RH. Extracorporeal life support: History and new directions. Semin Perinatol. 2005;29:2-7.
- Makdisi G, Wang I. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. Journal of Thoracic Disease. 2015;7:E166-E176.
- Nunes LB, Mendes PV, Hirota AS, Barbosa EV, Maciel AT, Schettino GP, et al. Severe hypoxemia during veno-venous extracorporeal membrane oxygenation: exploring the limits of extracorporeal respiratory support. Clinics. 2014;69:173-178.
- Donaldson AJ, Thomson HE, Harper NJ, Kenny NW. Bone cement implantation syndrome. Br J Anaesth. 2009;102:12-22.
- Urban MK, Sheppard R, Gordon MA, Urquhart BL. Right ventricular function during revision total hip arthroplasty. Anesth Analg. 1996;82:1225-1229.
- Byrick RJ. Cement implantation syndrome: A time limited embolic phenomenon. Can J Anaesth. 1997;44:107-111.
- Issack PS, Lauerman MH, Helfet DL, Sculco TP, Lane JM. Fat embolism and respiratory distress associated with cemented femoral arthroplasty. Am J Orthop. 2009;38:72-76.
- Heinrich H, Kremer P, Winter H, Wörsdorfer O, Ahnefeld FW, Wilder-Smith O. Embolic events during total hip replacement: an echocardiographic study. Acta Orthop Belg. 1988;54:12-17.
- Christie J, Burnett R, Potts HR, Pell AC. Echocardiography of transatrial embolism during cemented and uncemented hemiarthroplasty of the hip. Bone Joint J. 1994;76:409-412.
- Pitto RP, Koessler M, Draenert K. Prophylaxis of fat and bone marrow embolism in cemented total hip arthroplasty. Clin Orthop Relat Res. 1998;355:23-34.
- Koessler MJ, Fabiani R, Hamer H, Pitto RP. The clinical relevance of embolic events detected by transesophageal echocardiography during cemented total hip arthroplasty: A randomized clinical trial. Anesth Analg. 2001;92:49-55.
- Pagani FD, Aaronson KD, Swaniker F, Bartlett RH. The use of extracorporeal life support in adult patients with primary cardiac failure as a bridge to implantable left ventricular assist device. Ann Thorac Surg. 2001;71:S77-S81.
- Azevedo LCP, Park M, Costa ELV. Oxigenação extracorpórea por membrana na hipoxemia grave: hora de revermos nossos conceitos? J Bras Pneumol. 2012;38:7-12.
- Raoof S, Goulet K, Esan A, Hess DR, Sessler CN. Severe hypoxemic respiratory failure: part 2—nonventilatory strategies. Chest. 2010;137:1437-1448.
- Gail AM, Lynch WR, MacLaren G, Wilson JM, Bartlett RH. ECMO - Extracorporeal Cardiopulmonary Support in Critical Care. 4th ed. Ann Arbor, MI: Extracorporeal Life Support Organization (ELSO); 537 p. 2012.
- Abrams D, Brodie D. Extracorporeal circulatory approaches to treat acute respiratory distress syndrome. Clin Chest Med. 2014;35:765-779.
- Extracorporeal Life Support Organization. Elso Guidelines. Special Topic Guidelines. Elso Anticoagulation Guideline. 2014.
- Extracorporeal Life Support Organization. Elso Guidelines. Respiratory Support Guidelines. Guidelines for Adult Respiratory Failure. 2013.





 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.