Brainstem Auditory Evoked Responses with Duration of Type-II Diabetes Mellitus
2 Department. of Physiology, L.N. Medical College and J.K. Hospital, Bhopal, India, Email: arbindchoudhary111@gmail.com
Citation: Chhaya Batham, Arbind Kumar Choudhary, Praveen S. Yousuf, Brainstem auditory evoked responses with duration of type-II diabetes mellitus. Ann Med Health Sci Res. 2017; 7: 40-45.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Global incidence of Type-2 diabetes mellitus (T2DM) and the detrimental effects that it has on the hearing ability of individuals causes pathophysiological changes. With the help of evoked potential techniques, the brain stem auditory response represents a simple procedure to detect both acoustic nerve and central nervous system pathway damage. Objective: The present study was to investigate hearing function by brainstem evoked response audiometry (BERA) in well characterized type 2 Diabetic patients, with the role of potentially relevant factors such as duration of its disease and was target, these finding might help to determine early subclinical hearing impairment in these patients. Method: In clinical and laboratory setting, participants were divided randomly into following groups. Group I-(n=50) - control group and the study group were diabetic Individuals such as patients having diabetes less than or equal to five years ≤ 5 years) (n=29) and patients having diabetes more than five years ( > 5 years) (n=21) and were examined for BERA abnormalities (for 70, 80 and 90 db. Result: We observed a significant difference (‘p’<0.05) in BERA abnormalities (for 70, 80 and 90 db), in study group when compare to control. There were statistically significant delayed in absolute latencies of respective wave in both left ear and right ear, however interpeak latency side was comparable in both left and right ear. Conclusion: This study suggests that duration of diabetes may play a significant important role in the occurrence of diabetes mellitus associated hearing loss and patients may suffer from hearing impairment sooner or later, however if detected early further deterioration in auditory function can be prevented if not treated and also suggested that BERA testing may include in the routine screening procedures that are of vital importance in diabetic patients, wherever it is possible.
Keywords
BERA; Diabetes mellitus; Blood glucose; Hearing impairment
Introduction
Patients with Type-2 diabetes mellitus (T2DM) comprise of hyperglycemia with symptom of metabolic disorders and complications such as neuropathy being most frequent. [1].
Diabetic peripheral neuropathy in T2DM having a significant negative impact on quality of life. [2]. The diabetic neuropathies are present with diverse clinical manifestations. [3]. T2DM produces complications of neurologic malfunction and having hearing impairment greater than those without. [4].
The source of the pathophysiology of hearing deficit/ hearing loss, the location(s) of the lesions, and exact mechanism of these complications associated with diabetes mellitus has not been established up till now. However, during diabetes mellitus, associated pathophysiology change such as severity of serum glucose level, [5] elevated serum creatinine [6] thickened vessels of the stria vascularis, atrophy of the stria vascularis, and loss of outer hair cells in cochlea [7]. has been reported. There is usage of auditory brainstem response testing in the clinical evaluation (hearing deficit/ hearing loss) of the patients with diabetes mellitus and the likelihood of encountering a diabetic complication increases as the results brainstem response become abnormal. [8].
Diabetes mellitus (DM) produces complications of vascular and neurologic malfunction in those with the disorder. Diabetes mellitus (DM) produces complications of vascular and neurologic malfunction in those with the disorder. Diabetes mellitus (DM) produces complications of vascular and neurologic malfunction in those with the disorder. In diabetes mellitus, the auditory system shows some histological changes, such as; decrease in the number of ciliated cells, diffused thickening of the basilar membrane, atrophy of spiral ganglion and demyelination in the 8th nerve. [9]. There is involvement of the smaller vessels in the inner ear that leads to hypoxia and thus leading to hearing loss In diabetes mellitus. [10].
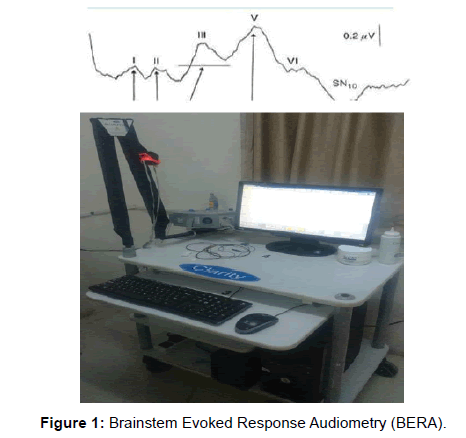
Brainstem Evoked Response Audiometry (BERA) were recorded from the vertex of human and wave latencies are mostly generated by brainstem structure located at a considerable distance from the recording point and useful in evaluating subcortical function. [11]. The various brainstem evoked response audiometry (BERA) components can be used to localize the auditory neuropathy to particular portions of the nerve. [12]. Wave I is produced in the distal portion of the nerve within the cochlea (VIII nerve); wave II is produced within the cochlear nucleus; wave III is produced; with in superior olivary nucleus wave IV is produced from lateral lemniscus and wave V is produced from Inferior colliculi. [13]. [Figure 1].
Method
The present study was approved by the local research advisory committee of LN Medical College and Research Centre (LNMC/Dean/2015/2146) and conducted in the Department of Physiology, LN Medical College and JK Hospital, India. The study was performed in accordance with the Declaration of Helsinki. In the clinical and audiometry laboratory settings, diabetic patients were examined using BERA testing and the results were interpreted. The study group (n=50) comprises of Type-2 diabetes patients and a total of n= 50 healthy volunteers were taken as control.
Study design
Inclusion and exclusion criteria
Inclusion criteria: (1) Subjects suffering from biochemically proved Diabetes mellitus (2) Age group between 30 - 65 years, of both patients and controls (3) Subjects of 0-20 years of Diabetes standing were divided into 2 groups (a) less than or equal to five years (≤ 5) years (b) more than five years (>5 years). Exclusion criteria: Patients who gave history of ear disease due to exposure to prolonged loud noise, intake of ototoxic drugs (ampicillin, chloroquine, metronidazole, and other drugs), stroke, head injury, or family history of deafness and other medications (methyldopa, reserpine, phenytoin, antipsychotic antidepressant, etc.), which interfere with the functioning, and DM neuropathy patients were also excluded from this study.
Participants
All selected participants were divided randomly into following groups. Group I-(n=50)- control group and the study group were diabetic Individuals such as patients having diabetes less than or equal to five years (≤ 5 years) (n=29) and patients having diabetes more than five years ( >5 years) (n=21).
Protocol
Estimation of blood glucose: Biochemically random blood glucose was estimated by GOD POD method to confirm Type- 2 diabetes. [14,15].
Recording of brainstem evoked response audiometry (BERA): The brain evoked response audiometry was obtained by employing a conventional far-field scalp averaging technique and the equipment used was RMS EMG Octopus by Clarity Medical Pvt. Ltd. with 2 amplifiers with hardware version 2.5 and software version 4.2. Earlier to the test, participants were instructed: to wash their hair to be oil free and the procedure was elucidated to the patient and asked to lie down comfortably on bed in relaxed state in a quiet and dimly lit room. Skin of the forehead and of mastoid process was cleaned with acetone soaked swab. Cleaned Electrodes (ground electrode: (Fz), reference Electrode (Cz): Vertex, active Electrode (Oz): mastoid process) were properly placed by using 10-20 conductive paste applied in the recess of electrode and then adhered to cleaned surface of their respective side. Standard silver chloride electrodes of 1 cm diameter were placed according to 10-20 International System. [16]. The stimulus was given using head phone. The stimulus rate was set at 11 clicks/sec., sweep speed was set at 1 ms/div., low filter was set at 100 Hz and high filter at 3 KHz. Recording were taken at Intensity of 70, 80, and 90 dB was used to determine threshold response with rare click stimulus. The impedance between skin and electrodes were kept less than 5 Kohm. Auditory stimuli consisting of rarefaction clicks of 100 microseconds were provided through electrically shielded earphones at a rate of 11.1/sec. Contralateral ear was masked with pure white noise of 40 dB. A band pass of 150- 3000 Hz was used to filter out undesirable frequencies in the surroundings. Averaging was done for 2000 epochs. Impedance was kept less than 5kΩ. At least 2 recordings were taken to confirm the re-producibility of wave form and the absolute latencies of wave I, III and V and interpeak latencies I-III, III-V, and I-V were recorded.
Calculation for sample size
A sample size of 96 was considered adequate for our study (we have studied 100 i.e., 50 diabetic and 50 control subjects). This was calculated by survey system (http://www.surveysystem. com/sscalc.htm#one). The mathematics of probability proves the size of the population is irrelevant unless the size of the sample exceeds a few percent of the total population we are examining. The survey system ignores the population size when it is “large” or unknown. Population size is only likely to be a factor when we work with a relatively small and known group of people (e.g. the members of an association).
Statistical Analysis
Data was entered in Microsoft excel 2010 and analysed using SPSS version 20 software for statistical analysis and students unpaired t-test was done. All the data was expressed as Mean and SD separately for right and left ear. Paired t-test was used for intergroup comparison. Qualitative data was presented with the help of frequency and percentage table. Association among the study groups was assessed with the help of chi-Square test. ‘p’ value less than 0.05 was taken as significant.
Results
Blood glucose level
Random blood glucose was observed to be significantly higher in diabetic patients groups when compared to control group.
Comparison of BERA parameters for 70 db, in absolute latencies (Wave I, II, II, IV, and V) and Inter-peak latency (I – III; I-V and III-V)
The data are summarized in Table 1 with Mean ± SD. Among diabetics <5 years as well as diabetics >5 years, when compare to control, we observed, there were statistically significant delayed in absolute latencies of wave I & III in both left ear and right ear and for wave V, only in left ear and for wave IV only in right ear. However, inter-peak latency (IPL) in both left and right side was comparable among both diabetic and controls groups.
| Side | Measurement | Control (50) | Diabetic ≤ 5 yrs (29) | Diabetic >5 yrs (21) | Control vs. Diabetic ≤ 5 yrs | Control vs. Diabetic >5 yrs | Diabetic ≤ 5 vs. diabetic > 5 yrs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t-value | P value | t-value | P value | t-value | P value | |||
| Left | Absolute latencies (ms) | I | 1.6 | 0.3 | 2 | 0.33 | 2.1 | 0.34 | 5.61 | 0.0001* | 6.35 | 0.0001* | 1.11 | 0.272 |
| II | 2.53 | 0.33 | 2.6 | 0.34 | 2.7 | 0.27 | 1.28 | 0.204 | 2.02 | 0.04 | 0.729 | 0.47 | ||
| III | 3.5 | 0.42 | 3.8 | 0.21 | 3.8 | 0.27 | 3.45 | 0.001* | 3.56 | 0.001* | 0.97 | 0.33 | ||
| IV | 4.5 | 0.31 | 4.4 | 0.44 | 4.4 | 0.46 | 0.551 | 0.582 | 0.417 | 0.67 | 0.06 | 0.95 | ||
| V | 5.2 | 0.39 | 5.7 | 0.39 | 5.7 | 0.2 | 5.23 | 0.0001* | 5.19 | 0.0001* | 0.08 | 0.932 | ||
| Inter-peak latencies (ms) | I-III | 1.9 | 0.52 | 1.84 | 0.6 | 1.8 | 0.42 | 0.654 | 0.515 | 0.898 | 0.372 | 0.2 | 0.83 | |
| I-V | 3.7 | 0.48 | 3.7 | 0.42 | 3.8 | 0.36 | 0.042 | 0.967 | 0.918 | 0.362 | 0.9 | 0.37 | ||
| III-V | 1.8 | 0.45 | 1.89 | 0.31 | 1.92 | 0.28 | 0.927 | 0.357 | 1.08 | 0.282 | 0.313 | 0.75 | ||
| Right | Absolute latencies (ms) | I | 1.6 | 0.25 | 1.86 | 0.4 | 1.85 | 0.31 | 3.19 | 0.002* | 3.12 | 0.002* | 0.12 | 0.9 |
| II | 2.6 | 0.34 | 2.6 | 0.37 | 2.7 | 0.33 | 0.78 | 0.43 | 0.845 | 0.401 | 0.101 | 0.92 | ||
| III | 3.5 | 0.39 | 3.8 | 0.36 | 3.8 | 0.29 | 4.47 | 0.0001* | 3.89 | 0.0001* | 0.27 | 0.78 | ||
| IV | 4.3 | 0.42 | 4.6 | 0.37 | 4.6 | 0.4 | 3.1 | 0.003* | 3.15 | 0.002* | 0.42 | 0.67 | ||
| V | 5.4 | 0.41 | 5.4 | 0.49 | 5.7 | 0.27 | 0.324 | 0.747 | 3.4 | 0.001 | 2.5 | 0.01 | ||
| Inter-peak latencies (ms) | I-III | 1.9 | 0.5 | 2.1 | 0.49 | 2.17 | 0.31 | 1.69 | 0.09 | 2.1 | 0.03 | 0.468 | 0.642 | |
| I-V | 3.8 | 0.42 | 3.8 | 0.33 | 3.8 | 0.34 | 0.034 | 0.97 | 0.35 | 0.72 | 0.417 | 0.678 | ||
| III-V | 1.9 | 0.53 | 1.93 | 0.45 | 2.05 | 0.49 | 0.077 | 0.939 | 0.84 | 0.402 | 0.901 | 0.372 | ||
Where *significance change (p<0.05) compared with control
Table 1: Latencies of BERA and duration of diabetes mellitus with 70 db.
Comparison of BERA parameters for 80 db, in absolute latencies (Wave I, II, II, IV, and V) and Inter-peak latency (I – III; I-V and III-V)
The data are summarized in Table 2 with Mean ± SD. We observed, there were statistically significant delayed in absolute latencies of wave I & IV only in the left ear, among diabetics <5 years as well as diabetics >5 years, when compare to control. However, for wave II, statistical significant difference was observed in both right and left ear, only for diabetics >5 years, when compare to control. Though, not any statistical significant difference was observed for other wave and inter-peak latency among both diabetic and controls groups.
| Side | Measurement | Control (50) | Diabetic ≤5 yrs (29) | Diabetic >5 yrs (21) | Control Vs Diabetic ≤5yrs | Control Vs Diabetic >5yrs | Diabetic ≤5 vs diabetic >5yrs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t-value | P value | t-value | P value | t-value | P value | |||
| Left | Absolute latencies (ms) | I | 1.6 | 0.19 | 1.5 | 0.2 | 1.59 | 0.19 | 2.08 | 0.04* | 0.173 | 0.86 | 1.51 | 0.13 |
| II | 2.62 | 0.3 | 2.5 | 0.34 | 2.4 | 0.24 | 1.63 | 0.12 | 2.63 | 0.01* | 0.83 | 0.4 | ||
| III | 3.51 | 0.2 | 3.4 | 0.42 | 3.3 | 0.43 | 1.41 | 0.16 | 1.61 | 0.11 | 0.177 | 0.86 | ||
| IV | 4.58 | 0.31 | 4.4 | 0.42 | 4.3 | 0.4 | 1.92 | 0.05* | 2.52 | 0.01* | 0.53 | 0.59 | ||
| V | 5.42 | 0.42 | 5.4 | 0.32 | 5.4 | 0.28 | 0.375 | 0.709 | 0.232 | 0.818 | 0.64 | 0.52 | ||
| Inter-peak latencies (ms) | I-III | 1.92 | 0.27 | 1.9 | 0.51 | 1.8 | 0.51 | 0.082 | 0.935 | 1.25 | 0.214 | 0.75 | 0.45 | |
| I-V | 3.8 | 0.46 | 3.9 | 0.41 | 3.8 | 0.37 | 1.34 | 0.18 | 0.108 | 0.915 | 1.34 | 0.188 | ||
| III-V | 1.9 | 0.47 | 2.05 | 0.4 | 2 | 0.38 | 1.43 | 0.156 | 0.844 | 0.4 | 0.45 | 0.65 | ||
| Right | Absolute latencies (ms) | I | 1.49 | 0.24 | 1.53 | 0.2 | 1.53 | 0.24 | 0.723 | 0.47 | 0.58 | 0.55 | 0.018 | 0.98 |
| II | 2.6 | 0.27 | 2.68 | 0.25 | 2.5 | 0.38 | 0.132 | 0.89 | 2.2 | 0.03* | 1.89 | 0.06 | ||
| III | 3.4 | 0.26 | 3.48 | 0.25 | 3.4 | 0.39 | 0.06 | 0.95 | 0.18 | 0.85 | 0.198 | 0.844 | ||
| IV | 4.47 | 0.33 | 4.43 | 0.44 | 4.56 | 0.48 | 0.44 | 0.64 | 0.923 | 0.359 | 0.99 | 0.327 | ||
| V | 5.45 | 0.32 | 5.52 | 0.33 | 5.4 | 0.28 | 0.87 | 0.38 | 0.332 | 0.74 | 1.04 | 0.301 | ||
| Inter-peak latencies (ms) | I-III | 1.98 | 0.36 | 1.9 | 0.37 | 1.93 | 0.6 | 0.28 | 0.77 | 0.36 | 0.72 | 0.123 | 0.902 | |
| I-V | 3.9 | 0.46 | 3.94 | 0.49 | 3.9 | 0.39 | 0.06 | 0.94 | 0.36 | 0.77 | 0.27 | 0.788 | ||
| III-V | 1.98 | 0.37 | 1.98 | 0.47 | 1.96 | 0.49 | 0.05 | 0.95 | 0.16 | 0.87 | 0.165 | 0.87 | ||
Where *significance change (p<0.05) compared with control
Table 2 : Latencies of BERA and duration of diabetes mellitus with 80 db.
Comparison of BERA parameters for 90 db in absolute latencies (wave I, II, II, IV, and V) and Inter-peak latency (I - III: I-V and III-V)
The data are summarized in Table 3 with Mean ± SD. there were statistically significant delayed in absolute latencies of wave IV only in the right ear, among diabetics <5 years as well as diabetics >5 years, when compare to control. However, in the left ear, it was observed for only for diabetics <5 years, when compare to control. In addition to, there was significant difference in the left ear for wave II, as well as Inter-peak latency I-III, among diabetics >5 years, when compare to control . Other wave and interpeak latency in both left and right side was comparable among both diabetic and controls groups.
| Side | Measurement | Control (50) | Diabetic ≤5 yrs (29) | Diabetic >5 yrs (21) | Control Vs Diabetic ≤5yrs | Control Vs Diabetic >5yrs | Diabetic ≤5 vs diabetic >5yrs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | t-value | P value | t-value | P value | t-value | P value | |||
| Left | Absolute latencies (ms) | I | 1.51 | 0.2 | 1.48 | 0.217 | 1.55 | 0.24 | 0.57 | 0.56 | 0.688 | 0.49 | 1.028 | 0.309 |
| II | 2.68 | 0.26 | 2.58 | 0.28 | 2.4 | 0.2 | 1.56 | 0.123 | 3.04 | 0.003* | 1.38 | 0.17 | ||
| III | 3.61 | 0.35 | 3.65 | 0.35 | 3.4 | 0.47 | 0.47 | 0.63 | 1.79 | 0.07 | 1.89 | 0.06 | ||
| IV | 4.4 | 0.24 | 4.5 | 0.23 | 4.43 | 0.51 | 2.41 | 0.01* | 0.178 | 0.85 | 1.1 | 0.27 | ||
| V | 5.47 | 0.39 | 5.34 | 0.255 | 5.18 | 1.09 | 1.63 | 0.107 | 1.68 | 0.09 | 0.77 | 0.44 | ||
| Interpeak latencies (ms) | I-III | 2.09 | 0.35 | 2.14 | 0.48 | 1.87 | 0.59 | 0.57 | 0.56 | 1.91 | 0.05* | 1.77 | 0.08 | |
| I-V | 3.9 | 0.38 | 3.86 | 0.41 | 3.8 | 0.41 | 0.925 | 0.35 | 0.655 | 0.51 | 0.15 | 0.87 | ||
| III-V | 1.85 | 0.45 | 1.69 | 0.33 | 1.98 | 0.54 | 1.6 | 0.112 | 1.06 | 0.29 | 2.33 | 0.02 | ||
| Right | Absolute latencies (ms) | I | 1.55 | 0.21 | 1.5 | 0.27 | 1.48 | 0.29 | 0.646 | 0.52 | 1.02 | 0.311 | 0.34 | 0.73 |
| II | 2.6 | 0.28 | 2.65 | 0.33 | 2.62 | 0.29 | 0.38 | 0.7 | 0.06 | 0.95 | 0.25 | 0.8 | ||
| III | 3.58 | 0.27 | 3.6 | 0.25 | 3.55 | 0.29 | 0.82 | 0.413 | 0.45 | 0.64 | 1.09 | 0.27 | ||
| IV | 4.57 | 0.24 | 4.4 | 0.32 | 4.2 | 0.43 | 2.5 | 0.01* | 4.2 | 0.0001* | 1.65 | 0.105 | ||
| V | 5.37 | 0.43 | 5.3 | 0.39 | 5.48 | 0.37 | 0.03 | 0.973 | 1.01 | 0.315 | 0.95 | 0.346 | ||
| Interpeak latencies (ms) | I-III | 2.05 | 0.35 | 2.11 | 0.32 | 2.05 | 0.44 | 0.712 | 0.479 | 0.036 | 0.97 | 0.56 | 0.57 | |
| I-V | 3.82 | 0.48 | 3.86 | 0.47 | 3.98 | 0.34 | 0.402 | 0.689 | 1.35 | 0.18 | 0.917 | 0.36 | ||
| III-V | 1.8 | 0.55 | 1.76 | 0.47 | 1.98 | 0.48 | 0.312 | 0.75 | 1.26 | 0.21 | 1.56 | 0.124 | ||
Where *significance change (p<0.05) compared with control
Table 3 : latencies of BERA and duration of diabetes mellitus with 90 db.
Discussion
In the absence of particular symptoms, a simple, non-invasive technique. T2DM individuals are more prone to develop sensorineural hearing impairment. Usually, it is suggested that Brainstem auditory evoked potential (BAEP) or BERA can demonstrate electrophysiologically any lesions from acoustic nerve to the brainstem and can be used in diabetics to show subclinical variances and central neuropathy. [17]. In one study, 152 out of 265 diabetes patients had bilateral symmetrical hearing impairment mainly for the high frequencies, so called sensory neural hearing impairment. [18]. BERA analysis on 20 insulin-dependent diabetes patients, concluded that the latency of wave III to be significantly delayed by 0.30 ms and wave V by 0.45 ms and IPL wave I-III was delayed by 0.24 ms and I-V delayed by 0.35 m, s with 70, 80, and 90 dB. [19]. Recording of BERA from scalp of 30 normo-acoustic, showed delay in peripheral transmission time (wave I) and central transmission time (wave I-V) in insulin-dependent diabetes individuals. [20]. BERA in T2DM individuals(n=20) showed the delay in latencies of wave III, IV, V, and IPL I-III by 0.32 ms, as well as I-V by 0.35 ms. [21]. In addition to, the wave V latency was found to be delayed in diabetes patients. [22]. Despite a number of studies on BAEP in diabetic patients with well- controlled disease, conflicting data still exists on a possible association between bilateral progressive high frequency hearing loss and diabetes. [23]. Researchers reported a higher incidence of hearing impairment among diabetics in comparison to the healthy individuals. [13,24]. T2DM result in changes of cochlea, such as thickening of the vessels of basilar membrane, atrophy of the stria vascularis and outer hair cell loss. [7]. Patients with severe peripheral neuropathy and retinopathy seem to have an increased risk of hearing impairment. However, though no association between duration, severity of diabetes and hearing impairment has been reported. [25]. Reduced hearing threshold was observed in 30 diabetic patients but could not observed relationship between duration of diabetes to level of hearing impairment. [26]. The overall finding seems to indicate a central disturbance of auditory pathway and the microvascular complications. Although duration of diabetes has not been interrelated with the degree of hearing impairment but may associate with prolonged auditory brainstem latencies.
This study was investigated hearing function in well characterized T2DM patients and evaluated the role of potentially relevant factors such as duration of disease and evaluation of these changes might help to determine early subclinical hearing impairment in these patients. Previously study had found no correlation between insulin dosage and family history of diabetes and hearing threshold. [27]. However, some of the previous study has been shown that hearing impairment in diabetes is related to duration of diabetes mellitus. [13]. Patients with short term diabetes mellitus had normal level of hearing, but in patients suffering from long term diabetes mellitus, there was a significant hearing impairment in the higher frequencies (4-8 KHz) as compared to the control group. [13]. Individuals with diabetic neuropathy have prolonged absolute latency of wave V, IPL of III-V and I-V as well as lowered V: I ratio. [28]. Furthermore absolute latencies of wave I and III were increased significantly in diabetics with more than 5 years duration than the non–diabetic controls. [28]. The delay in neural conductance along the auditory pathway was observed in 43 diabetic patients, with the help of their auditory brainstem response (ABR). The absolute latencies of wave I, III, and V were significantly prolonged in the diabetic individuals when compared with the healthy individuals. [23]. The degree of hearing impairment has been interrelated with duration of diabetes. Type 2 diabetic patients had significantly higher incidence hearing impairment, when compared with controls. Mean pure tone audiometry thresholds were greater in diabetics for all frequencies but however more clinically relevant has been observed at 6000 and 8000 Hz. Prolonged auditory brainstem response; wave V latencies in the diabetic group suggest retro-cochlear involvement. Age and duration of diabetes mellitus play a significant important role in the occurrence of diabetes mellitus associated hearing impairment. [29].
In our study, we have observed a significant difference in BERA abnormalities with duration of diabetes. The delayed absolute latencies as well as inter-peak latencies suggests abnormal neural firing synchronization or in the transmission in the auditory pathways in T2DM patients. [30]. Our findings are in covenant with previous studies and found a strong association of BAEP with duration of diabetes. [31,32]. From our study, we can say that duration of illness may be the risk factors for the development of central neuropathy in the diabetic patients.
Conclusion
This study was undertaken to assess the usefulness of BERA in early detection of hearing impairment in type 2 diabetic patients, and in this study, significant differences were observed in BERA latencies in T2DM patients when compare to healthy individuals, which indicate that patients with type 2 diabetes mellitus may suffer from hearing impairment sooner or later, however if detected early further deterioration in auditory function can be prevented if not treated. As diabetes is rampant in India, it is necessary to consider the “hearing status as a long term complication of diabetes. We recommended to perform a BERA test initially on all the diabetic patients and to keep this as an “initial record of auditory examination of patients”. Also, performing this test every year on a regular basis could help the physician to up-date their record of the hearing status of the patients as well as to give the necessary guidance in regard to the control of diabetes to them.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Sumner C, et al. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003; 60: 108-111.
- Davies M, et al. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes care 2006; 29: 1518-1522.
- Boulton AJ, et al. Diabetic neuropathies. Diabetes care, 2005; 28: 956-962.
- Frisina ST, et al. Characterization of hearing loss in aged type II diabetics. Hearing Research 2006; 211: 103-113.
- Hirose K. Hearing loss and diabetes: You might not know what you're missing. Annals of Internal Medicine 2008; 149: 54-55.
- Kakarlapudi V, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otology & Neurotology, 2003; 24: 382-386.
- Fukushima H, et al., Effects of type 2 diabetes mellitus on cochlear structure in humans. Archives of Otolaryngology–Head & Neck Surgery 2006; 132: 934-938.
- Bayazit Y, et al. Use of the auditory brainstem response testing in the clinical evaluation of the patients with diabetes mellitus. Journal of the Neurological Sciences 2000; 181: 29-32.
- Starr A. The neurology of auditory neuropathy. Sininger IA. Starr Auditory neuropathy, a new perspective on hearing disorders. San Diego: Singular Publishing Group 2001; 37-49.
- Rust KR, et al. Inner ear damage secondary to diabetes mellitus: II. Changes in aging SHR/N-cp rats. Archives of Otolaryngology–Head & Neck Surgery, 1992; 118: 397-400.
- Jewett DL, Romano MN, Williston JS. Human auditory evoked potentials: possible brain stem components detected on the scalp. Science 1970; 167: 1517-1518.
- Martin H, Pratt H, Schegler J. The origin and nature of the human auditory brainstem response wave II. Electroencephalogr Clin Neurophysiol 1996; 87: 420-424.
- Sharma A. A comparative study of brainstem evoked response audiometry in diabetic and non -diabetic subject 2012.
- Rasmussen JB, et al. Random blood glucose may be used to assess long-term glycaemic control among patients with type 2 diabetes mellitus in a rural African clinical setting. Tropical Medicine & International Health 2014; 19: 1515-1519.
- Trinder P. Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. Journal of Clinical Pathology 1969; 22: 158-161.
- Kalita J, Misra U, Das M. Neurophysiological criteria in the diagnosis of different clinical types of Guillain–Barré syndrome. Journal of Neurology, Neurosurgery & Psychiatry 2008; 79: 289-293.
- Markand ON. Brainstem auditory evoked potentials. Journal of Clinical Neurophysiology 1994; 11: 319-342.
- Rosen Z, Yanko L, Cohen A. Diabetic labyrinthopathy and retinopathy: A correlative study. Israel Journal of Medical Sciences 1972; 8: 781.
- Donald M, et al. Delayed auditory brainstem responses in diabetes mellitus. Journal of Neurology, Neurosurgery & Psychiatry 1981; 44: 641-644.
- Fedele D, et al. Impaired auditory brainstem-evoked responses in insulin-dependent diabetic subjects. Diabetes 1984; 33: 1085-1089.
- Mehra YN, Brain SY. Stem evoked response Audiometry in diabetes Mellitus. Indian J Otolaryngol 1987; 39: 163–166.
- Virtanierni J, Laakso M, Nuutinen J. Karjalainens Scott Brown's Otolaryngology. London: Butterworths, 1997; 6: 95.
- Durmus C, Yetiser S, Durmus O. Auditory brainstem evoked responses in insulin-dependent (ID) and non-insulin-dependent (NID) diabetic subjects with normal hearing. International Journal of Audiology 2004; 43: 29-33.
- Kurien M, Thomas K, Bhanu T. Hearing threshold in patients with diabetes mellitus. The journal of laryngology & Otology 1989; 103: 164-168.
- Duck SW, et al. Interaction between hypertension and diabetes mellitus in the pathogenesis of sensorineural hearing loss. The Laryngoscope 1997; 107: 1596-1605.
- Kurien M, Thomas K, Bhanu T. Hearing threshold in patients with diabetes mellitus. The journal of laryngology & Otology 1989; 103: 164-168.
- Klagenberg KF, et al. Vestibulocochlear manifestations in patients with type I diabetes mellitus. Revista Brasileira de Otorrinolaringologia 2007; 73: 353-358.
- Das T, et al. Studies on central nervous system function in diabetes mellitus. Journal of the Indian Medical Association 2001; 99: 84: 86-77, 89.
- Akinpelu OV, Mujica-Mota M, Daniel SJ. Is type 2 diabetes mellitus associated with alterations in hearing? A systematic review and meta-analysis. The Laryngoscope 2014; 124: 767-776.
- Mahallik D, Sahu P, Mishra R. Evaluation of auditory brain-stem evoked response in middle: Aged type 2 diabetes mellitus with normal hearing subjects. Indian Journal of Otology 2014; 20: 199.
- Gupta R, et al. Type-2 diabetes mellitus and auditory brainstem responses-A hospital based study. Indian Journal of Endocrinology and Metabolism 2010; 14: 9.
- Tay H, et al. Diabetes mellitus and hearing loss. Clinical Otolaryngology 1995; 20: 130-134.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.