Central Diabetes Insipidus, Central Hypothyroidism, Renal Tubular Acidosis and DandyŌĆæWalker Syndrome:New Associations
- *Corresponding Author:
- Dr. Abdulmoein Eid Al-Agha
Department of Pediatrics, Faculty of Medicine, King Abdulaziz University, P.O. Box 80215, Jeddah 21589, Saudi Arabia.
E-mail: aagha@kau.edu.sa
Abstract
Dandyâ├āŌĆÜ├éŌé¼├āŌĆÜ├éŌĆśWalker syndrome (DWS) is a rare brain malformation involving the cerebellum, and the fluid filled spaces around it, usually detected during the antenatal period or the early infancy. Clinically, it is characterized by mental retardation, developmental delay as well as cerebellar ataxia. It has been frequently associated with other conditions such as congenital heart diseases, primary hypothyroidism, and other disorders of the central nervous, gastrointestinal, genitourinary, and orthopedic systems. In this report, we describe a 3â├āŌĆÜ├éŌé¼├āŌĆÜ├éŌĆśmonthâ├āŌĆÜ├éŌé¼├āŌĆÜ├éŌĆśold Saudi boy with the rare association of DWS with central diabetes insipidus, congenital central hypothyroidism, and typeâ├āŌĆÜ├éŌé¼├āŌĆÜ├éŌĆś2 renal tubular acidosis.
Keywords
Central, Dandy-Walker, Diabetes insipidus, Hypothyroidism, Renal, Tubular acidosis
Introduction
Dandy-Walker syndrome (DWS) is a rare brain congenital malformation and genetically sporadic condition usually detected during the antenatal period. It is characterized by agenesis or hypoplasia of the cerebellar vermis, cystic dilatation of the fourth ventricle, and enlargement of the posterior fossa. [1]
Different nonrelated associations with DWS have been described in the literature before. [2,3] In this case report, we describe new associations for DWS: Central diabetes insipidus (CDI), renal tubular acidosis (RTA), and central hypothyroidism.
Case Report
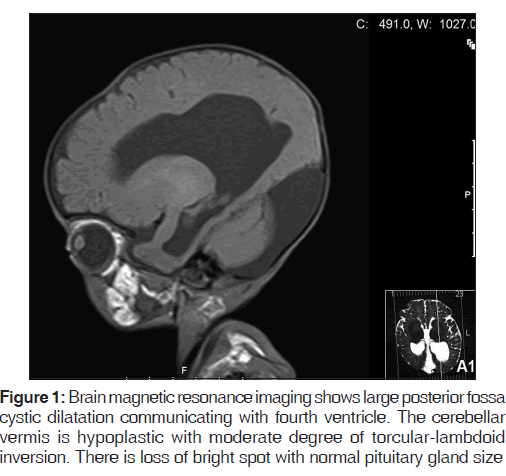
A 3-month-old, full-term boy was delivered by cesarean section to a 43-year-old female; he had a birth weight of 3.2 kg. Antenatal ultrasound showed hydrocephalus, which was confirmed postnatally. At the age of 2 weeks, he was readmitted to the hospital because of jaundice and failure to thrive, for which he was investigated and diagnosed to have central congenital hypothyroidism. Shortly thereafter, he was admitted to our institute with a history of vomiting, decreased oral intake, polyuria, and dehydration having lasted 5 days. He was investigated [Table 1], and diagnosed with CDI because of polyuria, a high urine output (UOP) of 12.5 cc/kg/h with a negative fluid balance of 55 ml/24 h, and hypernatremia. A water deprivation test could not be performed as the patient was dehydrated most of the time, with high levels of serum sodium. Brain magnetic resonance imaging (MRI) confirmed the Dandy-Walker malformation [Figure 1].
On physical examination, the patient’s vitals were stable. Initially, he was dehydrated, pale, with dry skin, a weight of 2.4 kg (<3rd centile), length of 52 cm (<3rd centile), and a head circumference of 40 cm (<25th centile). The patient had a widely opened anterior fontanelle as well as an opened posterior fontanelle. Neurological examination showed generalized hypotonia, hyperreflexia, decreased power and horizontal nystagmus. Developmentally, he was globally delayed. Other systemic examinations were unremarkable.
During hospitalization, he was managed with “Minirin Melt,” the oral form of the synthetic arginine vasopressin analogue “desmopressin,” after several titrations, a dose of 30 mcg TID was administered through a nasogastric tube 15 min prior to feeding. He was tolerating the treatment well, with a fairly good clinical and laboratory response, in addition to thyroxin replacement for his central hypothyroidism. A urinary catheter was inserted throughout the hospitalization to measure UOP. His fluid was calculated initially on his maintenance and UOP. At the polyuria stage, his maintenance was given as dextrose 5% in 0.2 normal saline (NS) in addition to UOP replacement of 0.45 NS (if > 3 ml/kg/h then ringer lactate was used). After UOP was normalized, fluid was given for maintenance only.
Despite remarkable improvement in the clinical manifestations of his polyuria and hydration status, he experienced a persistent metabolic acidosis with alkaline urine, glycosuria, proteinuria, and a negative urine culture [Table 1]. RTA was diagnosed, and the patient was administered oral indomethacin and bicarbonate to treat it.
Throughout his desmopressin treatment, the patient’s serum sodium remained fairly stable, but his weight gain was rather slow, as expected due to his other associated anomalies and the presence of RTA. Follow-up metrics during his admission were based on daily urea and electrolytes, blood gas, UOP, and weekly anthropometric measurements. In the 1st month of his discharge, he was seen on a weekly basis, and then monthly. In each follow-up visit, his hydration status was evaluated, as was his weight, serum sodium and osmolality, and urine sodium and osmolality.
Discussion
Dandy-Walker syndrome is a rare malformation characterized by specific structural imaging changes. [1] In the present report, brain MRI showed some related structural changes [Figure 1]. Affected patients can present with a developmental motor delay, increased intracranial pressure and signs of cerebellar dysfunction. [1] We described similar findings in our case, including global developmental delay, hypotonia, and nystagmus. The etiology is not clearly defined, but some nonrelated systemic disorders frequently associated with DWS have been described. [2,3] This patient had central hypothyroidism based on a low free thyroxin level together with low thyroid stimulating hormone [Table 1]. In the literature, primary hypothyroidism was found to be associated with DWS.[2] In a case series of 24 infants and children with DWS, some cases were found to be associated with hydrocephalus; however, most of them had other central nervous system anomalies.[3] Similar findings were described in our case as well. CDI was clinically diagnosed in our case with polyuria and hypernatremia, with a good response to desmopressin supplementation. Some cerebral malformations have been associated with CDI, but DWS has not been reported previously in the literature. A relationship between CDI and hydrocephalus, however, has been reported before.[4] The treatment of CDI with desmopressin is challenging in neonates and infants, and there is insufficient literature regarding its dosage or route of administration. Previously, desmopressin has been ordered in managing CDI in young children using different routes of administration.[4,5] A retrospective study concluded that the introduction of oral desmopressin in early infancy for the treatment of CDI is safe and practical.[5] We used the oral form of desmopressin (desmopressin lyophilisate), a dose of 60 mcg/tablet dissolved in water (3–5 ml) was initiated with a dose of 10 mcg/kg/dose three times daily together with limitation of water intake to avoid hyponatremia. The administration was under continuous monitoring and follow-up, besides titrating the dose according to the clinical and laboratory parameters. On the other hand, RTA was diagnosed, with persistent metabolic acidosis, alkaline urine, proteinuria, and glycosuria despite correction of the fluid balance and hydration after starting the treatment [Table 1]. In comparison to the available reports of DWS, no other such association has been described. CDI and central hypothyroidism could be explained by the occurrence of hydrocephalus due to DWS. However, there is no clear explanation for its association with RTA.
| Investigations done | Pretreatment | Posttreatment | Normal range |
|---|---|---|---|
| Serum sodium | 161 | 143 | 135-145 mmol/L |
| Serum osmolality | 338 | 292 | 275-295 mos/kg |
| Urine osmolality | 186 | 310 | 50-1400 mos/kg |
| Urine specific | 1.005 | 1.015 | 1.005-1.020 |
| gravity | |||
| Serum potassium | 3.4 | 3.6 | 3.5-5.1 mmol/L |
| Serum urea | 12.1 | 5.5 | 2.5-6.4 mmol/L |
| Serum cortisol | 754 | 754 | 138-636 nmol/L |
| Serum glucose | 3.9 | 5.1 | 3.9-6.7 mmol/L |
| pH | 7.02 | 7.3 | 7.35-7.45 |
| HCO3 | 10 | 20 | 19.8-24.2 mmol/L |
| BE | −13 | −2 | 2- (−2) |
| Urine glucose | +++ | ++ | - |
| Urine proteins | ++ | ++ | - |
| Urine pH | 9 | 6 | - |
| Urine culture | No growth | ||
| FT4 | 9.98 | - | 12-22 pmol/L |
| FT3 | 2.22 | - | 2.8-7 pmol/L |
| TSH | 0.13 | - | 0.27-4.2 uIU/L |
| ACTH | 1.76 | - | 1.6-13.9 pmol/L |
TSH: Thyroid stimulating hormone, ACTH: Adrenocorticotropic hormone, BE: Base excess
Table 1: Laboratory investigations
Conclusion
In this report, we described a new association with DWS occurring in a Saudi boy, which has not been previously reported in the literature. The identification of such associations leads to immediate care of patients and highlights the importance of a complete evaluation, including imaging studies, when presented with an infant or child with similar symptoms.
References
- Notaridis G, Ebbing K, Giannakopoulos P, Bouras C, Kövari E. Neuropathological analysis of an asymptomatic adult case with Dandy-Walker variant. Neuropathol Appl Neurobiol 2006;32:344-50.
- Ozdemir O, Polat A, Cinbis M, Kurt F, Kucuktasci K, Kiroglu Y. Dandy-Walker’s variant and tetralogy of Fallot with atrial septal defect and patent ductus arteriosus and primary hypothyroidy – a new association. Indian J Pediatr 2009;76:433-5.
- Sasaki-Adams D, Elbabaa SK, Jewells V, Carter L, Campbell JW, Ritter AM. The Dandy-Walker variant: A case series of 24 pediatric patients and evaluation of associated anomalies, incidence of hydrocephalus, and developmental outcomes. J Neurosurg Pediatr 2008;2:194-9.
- Van der Kaay DC, Van Heel WJ, Dudink J, van den Akker EL. Transient diabetes insipidus in a preterm neonate and the challenge of desmopressin dosing. J Pediatr Endocrinol Metab 2014;27:769-71.
- Korkmaz HA, Demir K, Kiliç FK, Terek D, Arslanoglu S, Dizdarer C, et al. Management of central diabetes insipidus with oral desmopressin lyophilisate in infants. J Pediatr Endocrinol Metab 2014;27:923-7.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.