Central Line Associated Blood Stream Infection Rate after Intervention and Comparing Outcome with National Healthcare Safety Network and International Nosocomial Infection Control Consortium Data
- *Corresponding Author:
- Dr. Syed Zahid Bukhari
Department of Infection Prevention and Control, Hera General Hospital, P.O. Box 20865, Makkah 10513, Kingdom of Saudi Arabia.
E-mail: zahidbukhari100@msn.com
Abstract
Background: Benchmarking of central line associated blood stream infection (CLABSI) rates remains a problem in developing countries due to the variations in surveillance practices and/or infection risk as non‑availability of national data. Aim: The aim of the following study was to find out the CLABSI rate before and after central line (CL) bundle intervention and compare the outcome with international surveillance data. Subjects and Methods: This prospective longitudinal cohort study on adult intensive care unit patients was conducted at Hera General Hospital, Makkah Saudi Arabia from January 1 to December 31, 2012. Five key components of bundle were selected; hand hygiene, maximal barrier precautions upon insertion, skin antisepsis, optimum site selection and daily review of line necessity with prompt removal of unnecessary lines. Post‑intervention CLABSI rate was compared with National Healthcare Safety Network (NHSN) and International Nosocomial Infection Control Consortium (INICC) rates. Statistical Package for the Social Sciences (SPSS) 14.0 software (SPSS Inc., 233 South Wacker Drive, 11th floor Chicago, USA) was used for statistical analysis included regression analysis for correlation. Statistical significance was set at P < 0.05. Results: CLABSI rate was reduced from 10.1 to 6.5 per 1000 CL days after interventions and had significant correlation with overall bundle compliance rate 87.6% (P = 0.02) On benchmarking, CLABSI rate after the intervention was similar to mean pool value of INICC (6.8) while higher than NHSN (3.1). The most common microorganisms isolated were; methicillin‑resistant Staphylococcus aureus (30.8%), Acinetobacter baumanii (23.3%) and Enterococcus faecalis (15.4%). Conclusion: We found that INICC data was a better benchmarking tool comparative to NHSN because it represents the countries that are developing the surveillance system. A multicenter national study is recommended.
Keywords
Central line associated blood stream infection, National healthcare safety network, Nosocomial infection control consortium, Saudi Arabia
Introduction
Healthcare associated infections (HAIs) occur throughout the world, affecting millions of patients each year.[1] Central line associated blood stream infections (CLABSI) can be prevented by evidence-based central line (CL) insertion and maintenance practices as a method for quality improvement opportunities and strategically targeting interventions.[2] The comparison of CLABSI rate remains a dilemma when national HAI surveillance data is not available and it may be due to variations in surveillance practices, infection risks, socio-economic conditions and high CLABSI rate reported locally.[3]
The current single center study was aimed to (1) find out CLABSI rate before and after intervention of CL bundle; (2) comparison of outcome with international surveillance data; National Healthcare Safety Network (NHSN)[4] and International Nosocomial Infection Control Consortium (INICC)[5] and (3) to identify microorganism profile of HAIs related to the CL.
Subjects and Methods
It is a prospective longitudinal cohort study conducted at medical and surgical adult intensive care unit (MSICU) patients at Hera General Hospital (HGH), Makkah. Saudi Arabia during 1 year period from January 1 to December 31, 2012. The HGH is a Joint Commission International accredited secondary acute healthcare facility with 280 beds capacity including 20 beds adult MSICU. The study was initiated as a quality improvement project to reduce CLABSI rate in ICU. The study was selected in response to annual infection control risk assessment where high CLABSI rate was one of the five top scored risks of infection prevention control in the hospital. This risk assessment was done by the multi-disciplinary team representing infection control practitioners, physicians, nurses and quality designees of ICU. All patients with CL insertion done in ICU were included while insertion outside ICU was the exclusion criteria of the study. There were two groups of patients; before and after interventions. For case finding, clinical examination and laboratory reports were followed. Microbiology tests were performed by the standard methods by using the Clinical Laboratory Standards Institute (CLSI) guideline.[6]
An active surveillance protocol was applied by the trained personnel used standard methodologies as described in Center for Disease Control and Prevention (CDC) guideline.[4] In current study, the CLABSI was defined as a primary laboratory confirmed blood stream infection in patients with a CL at the time of (or within 48-h prior to) the onset of symptoms and the infection is not related to an infection from another site. The infection cannot be related to any other infection the patient might have and must not have been present or incubating when the patient was admitted to the hospital. There was no minimum duration that the CL must be in place in order for the bloodstream infection to be considered CL associated.[5] The following sources of information were used for data surveillance collection; microbiology culture results, open or closed medical record review for any rise of temperature, change of antibiotics and physician/nurses progress notes. When assessing each positive blood cultures, the treating physician was sure that there was no other CDC-defined primary site of HAI that may have seeded the blood stream secondarily; otherwise the blood stream infection may be misclassified as a primary BSI or erroneously associated with the use of a CL, i.e., called a CLABSI.[5] The CLABSI rate was defined as the number of CL catheter-related blood stream infections per 1000 CL days. The regulation was set in such a way that even single missing element of bundles either not done, or are not documented, the whole bundle was considered not applied.[7] Before application of bundle intervention, it was assured that ICU had a written CL bundle policy in place; compliance was monitored; if so, how often compliance was observed. We also established a CL cart to consolidate all necessary items used in CL insertion and checklist was developed to ensure adherence to proper practice. The competency assessment of ICU privileged staff was done prior to implementation of CL bundle after education session in three shifts. The competency assessment process was completed in two sessions. In the second session includes those staff who did not qualify the first session. The CLABSI surveillance form was used to collect all required information.
The CL bundle insertion protocol was strictly implemented upon insertion and documented in checklist.[8] The following five key components were applied: (1) Hand hygiene performed with hospital-approved alcohol-based product or antiseptic-containing soap before and after palpating insertion sites and before and after inserting the CL; (2) maximal barrier precautions upon insertion were taken by the CL team comprised of a doctor and staff nurse assisting used sterile gown, sterile gloves, surgical mask and head caps. The patient was covered large sterile drape from head to toe only the area of insertion was exposed; (3) skin antisepsis was done by using swab stick impregnated with 2% W/V chlorhexidine gluconate and 70% V/V isopropyl alcohol. It was ensured that the skin preparation agent was completely dried before inserting the CL; (4) optimum site selection with avoidance of the femoral vein for central venous access in adult patients. It was done where the catheter was safely inserted and where the risk for infection is small and upper limb veins were preferred. The following great vessels were selected for CL insertion in adult ICU, internal jugular veins and subclavian veins; (5) daily review of line necessity with prompt removal of unnecessary lines were carried out.
The CL maintenance protocol[9] was followed throughout the placement period of CL which includes: (1) Pay attention to the bandage and the area around it; if the bandage comes off or if the bandage or area around it is wet or dirty, tell a healthcare worker right away; (2) do not get the CL or the CL insertion site wet; (3) tell a healthcare worker if the area around the catheter is sore or red or if the patient has a fever or chills; (4) do not touch catheter or tubing and do not let any visitors touch the catheter or tubing; (5) all visitors and care givers were instructed to sanitize hands before and after entering the patient’s room upon each visit. The microbiology profile included the organism identified in each CLABSI and antimicrobial susceptibility testing. In the first analysis, individual bundle component was evaluated based on compliance and calculated the overall CL bundle compliance rate. Second analysis was carried out to calculate the CLBSI rate. Third analysis was to test the effect of CL bundle as a whole to the reduction of CLABSI rate; individual bundle component was not tested to monitor the effect on CLABSI rate and fourth analysis was done by comparing data of with pre-intervention period. The CLABSI rate of year 2012 after the intervention was compared year 2011 data collected retrospectively before intervention. The outcome after the intervention was benchmarked with mean pool values of the two international organizations; NHSN[9] and INICC.[10] The target of CL bundle compliance was set on 100%.
Ethics
This study was been approved by the Ethical Committee of Hospital. All procedures done in the current study were reviewed and approved by Infection Prevention and Control Committee of the hospital. The general patient consent for any procedure in ICU was taken upon admission as per hospital policy and documented on each patient’s medical record. The outline of the uniform procedure was provided to the staff of concern units.
Statistical analysis
The data analysis, basic statistic, regression analysis for correlation and confidence interval (CI) was carried out on Statistical Package for the Social Sciences (SPSS) 14.0 student version ( SPSS Inc. 233 South Wacker Drive, 11th floor Chicago, USA). Statistical significance was set at P < 0.05.
Results
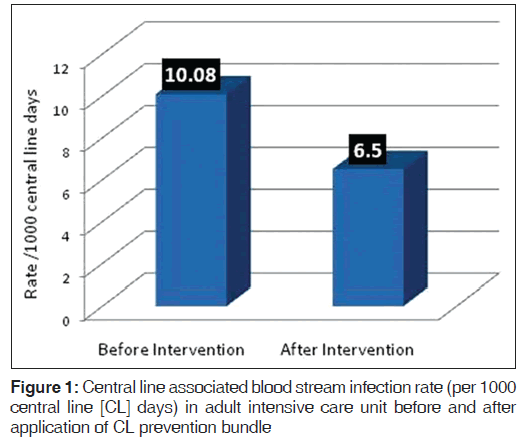
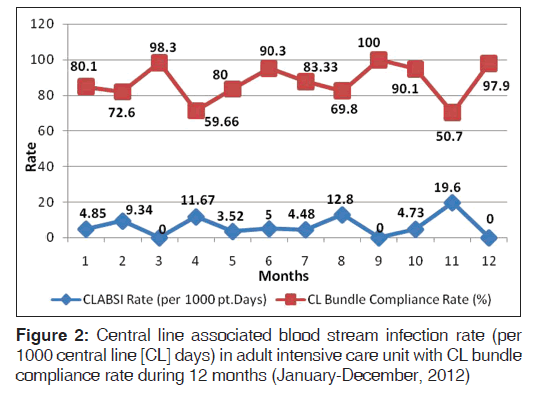
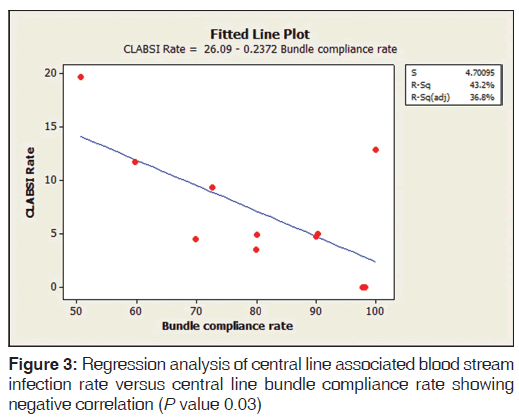
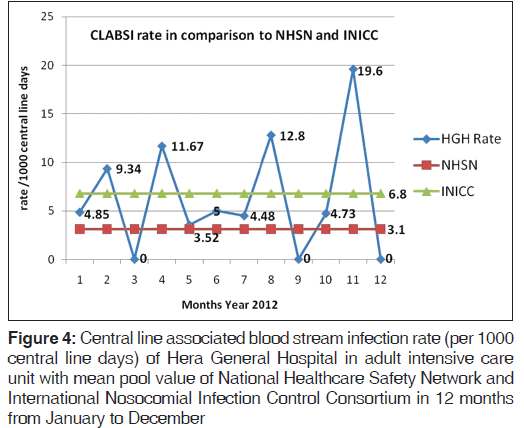
A total of 97 patients on CL in ICU were enrolled in the study. The average stay of CL in ICU was 7 days (range 3-21 days). The overall CLABSI rate was 6.5 (range 3.5-19.6) per 1000 CL days. There was 55.1% reduction in CLABSI after CL bundle intervention comparative to the base line rate before intervention. Figure 1 shows the CLABSI rate per 1000 CL days before and after intervention. The overall CL bundle compliance rate including five key components was 81.1%. Figure 2 illustrates CLABSI rate (per 1000 CL days) in adult ICU and central line bundle compliance rate during 12 months January-December, 2012 (P = 0.02). CI was 95%. Figure 3 illustrates regression analysis of CLABSI rate versus CL bundle compliance rate showing negative correlation. Figure 4 shows CLABSI rate in adult ICU of the current study in comparison to the mean pool values of two international HAI surveillance data; NHSN[9] and INICC.[10]
The microorganism profile of CLABSI in ICU was as follows; Methicillin-resistant Staphylococcus aureus 30.8% (4/13) followed by Acinetobacter baumanii 23.3% (3/13), Enterococcus faecalis 15.4% (2/13), Klebsiella pneumonia 7.7% (1/13), Serratia marcescans 7.7% (1/13) Escherichia coli 7.7% (1/13) and Staphylococcus aureus 7.7% (1/13).
Discussion
The CLABSI rate varies widely in healthcare facilities and reflects the infection prevention and control practice.[11] Initially, the CL team started this intervention as a quality improvement project to meet the challenge of reduction of CLABSI rate as described in other studies[12,13] and later continued as part of routine practice in ICU. The analysis of our data showed that overall CLABSI rate in ICU was significantly reduced after intervention (P = 0.02) comparable to many other similar studies[14,15] Our CLABSI rate was higher than El-Kholy et al.[16] where the CL bundle was applied 1st time as a quality improvement project while lesser than other similar study.[17]
In comparison to local studies, one study[3] reported higher CLABSI rate while in other study[18] it was lower than the current study. When we compared our data internationally with NHSN, we found our CLABSI rate is a higher than NHSN similar to other studies[2,19,20] while closer to INICC similar to other study.[21] The INICC data represents the countries which are developing the surveillance of HAIs[9] while NHSN data more or less belonged of the developed countries.[22] Similar differences were also published in other device associated infections in ICUs.[23]
CL bundle compliance rate remained 19% less than the target (100%) due to the following possible reasons; (1) double bed ICU with less space between the two beds; (2) non-availability of CL sterile full body drapes; (3) overcrowding; the bed occupying capacity remained more than 100%; (4) shortage of nursing staff; (5) incomplete documentation; (6) lack of knowledge of CL bundles especially when it is inserted during off working hours; (7) ignorance of staff; (8) temporary extension of ICU to the adjacent non-ICU unit during the month of Muslim pilgrimage[24] (hajj) where more than 3 million Muslims visited the place of study. This increase in ICU beds is to accommodate patients transferred from the other temporary hospitals established at pilgrimage areas only during Hajj period and the ratio of ICU patient and nurse become 1:3 which may lead to incomplete documentation. Therefore, authors recommend to hire more ad hoc employees in ICUs especially during hajj period.
There were certain limitations to this study. Firstly at the start of study, there was under reporting of CLABSI while over reporting CL bundle compliance which was discovered during the onsite validation visits and retrospective medical record review by the trained infection control practitioners similar to other studies.[25,26] The only reason of discrepancy was late filling up the CL bundle form. Therefore, the in-charge nurse in each shift was assigned to observe directly the compliance of key components and review reported data immediate after insertion. Secondly, incorporating the CL bundle intervention quality improvement project into a routine care practice was difficult because sudden withdrawal of quality improvement CL bundle team after completion of project and re-education of intensive care staff.
Conclusion
Higher adherence to the CL bundle intervention was positively correlated to a reduction in the CLABSI rate in ICU. We found that INICC data was better benchmarking tool comparative to NHSN as it represents the countries that are developing the surveillance system like in Saudi Arabia. A country wide multi-center study is recommended to formulate the national policy for benchmarking the surveillance outcome of device associated infections in ICUs.
Acknowledgments
The authors are thankful to the CL team included Dr. Samih Chakalli Consultant ICU, Dr. Saleh Abdorubo ICU Quality Designee, Roaa Basri Infection Control Practitioner, Fatima Harriri, Chief Lab. Technician, Liza Baylon CSSD Supervisor, Afaf Ireni Senior Microbiology Technician and Rhaiza Occubilo Infection Control Practitioner, No additional funding was required for the study as it was quality improvement project and part of the patient care.
References
- Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control 2007;35 (10 Suppl 2):S65-164
- Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, Pollock DA, et al. National Healthcare Safety Network (NHSN) Report, data summary for 2010, device-associated module. Am J Infect Control 2011;39:798-816.
- Al-Tawfiq JA, Amalraj A, Memish ZA. Reduction and surveillance of device-associated infections in adult intensive care units at a Saudi Arabian hospital, 2004-2011. Int J Infect Dis 2013;17:e1207-11.
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, et al. Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 2011;39:S1-34.
- Rosenthal VD, Maki DG, Graves N. The International Nosocomial Infection Control Consortium (INICC): Goals and objectives, description of surveillance methods, and operational activities. Am J Infect Control 2008;36:e1-12.
- Clinical and Laboratory Standards Institute (CLSI) Guideline 2011; M100-S21.
- Miller MR, Griswold M, Harris JM 2nd, Yenokyan G, Huskins WC, Moss M, et al . Decreasing PICU catheter-associated bloodstream infections: NACHRI’s quality transformation efforts. Pediatrics 2010;125:206-13.
- Jeong IS, Park SM, Lee JM, Song JY, Lee SJ. Effect of central line bundle on central line-associated bloodstream infections in intensive care units. Am J Infect Control 2013;41:710-6.
- D u d e c k M A , H o r a n T C , P e t e r s o n K D , Allen-Bridson K, Morrell GC, Pollock DA, et al. National Healthcare Safety Network (NHSN) report, data summary for 2009, device-associated module. Am J Infect Control 2011;39:349-67.
- Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control 2012;40:396-407.
- Thompson ND, Yeh LL, Magill SS, Ostroff SM, Fridkin SK. Investigating systematic misclassification of central line-associated bloodstream infection (CLABSI) to secondary bloodstream infection during health care-associated infection reporting. Am J Med Qual 2013;28:56-9.
- Bizzarro MJ, Sabo B, Noonan M, Bonfiglio MP, Northrup V, Diefenbach K, et al. A quality improvement initiative to reduce central line-associated bloodstream infections in a neonatal intensive care unit. Infect Control Hosp Epidemiol 2010;31:241-8.
- Huskins WC. Quality improvement interventions to prevent healthcare-associated infections in neonates and children. Curr Opin Pediatr 2012;24:103-12.
- Goeschel CA, Weiss WM, Pronovost PJ. Using a logic model to design and evaluate quality and patient safety improvement programs. Int J Qual Health Care 2012;24:330-7.
- Render ML, Hasselbeck R, Freyberg RW, Hofer TP, Sales AE, Almenoff PL, et al. Reduction of central line infections in Veterans Administration intensive care units: An observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf 2011;20:725-32.
- El-Kholy A, Saied T, Gaber M, Younan MA, Haleim MM, El-Sayed H, et al. Device-associated nosocomial infection rates in intensive care units at Cairo University hospitals: First step toward initiating surveillance programs in a resource-limited country. Am J Infect Control 2012;40:e216-20.
- Backman LA, Melchreit R, Rodriguez R. Validation of the surveillance and reporting of central line-associated bloodstream infection data to a state health department. Am J Infect Control 2010;38:832-8.
- Al-Tawfiq JA, Abed MS, Memish ZA. Peripherally inserted central catheter bloodstream infection surveillance rates in an acute care setting in Saudi Arabia. Ann Saudi Med 2012;32:169-73.
- Fontela PS, Platt RW, Rocher I, Frenette C, Moore D, Fortin E, et al. Epidemiology of central line-associated bloodstreaminfections in Quebec intensive care units: A 6-year review. Am J Infect Control 2012;40:221-6.
- Hu B, Tao L, Rosenthal VD, Liu K, Yun Y, Suo Y, et al. Device-associated infection rates, device use, length of stay, and mortality in intensive care units of 4 Chinese hospitals:International Nosocomial Control Consortium findings. AmJ Infect Control 2013;41:301-6.
- Furuya EY, Dick A, Perencevich EN, Pogorzelska M, Goldmann D, Stone PW. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One 2011;6:e15452.
- Rasslan O, Seliem ZS, Ghazi IA, El Sabour MA, El Kholy AA,Sadeq FM, et al. Device-associated infection rates in adult and pediatric intensive care units of hospitals in Egypt. International Nosocomial Infection Control Consortium (INICC) findings. J Infect Public Health 2012;5:394-402.
- Rasslan O, Seliem ZS, Ghazi IA, El Sabour MA, El Kholy AA, Sadeq FM, et al. Device-associated infection rates in adult and pediatric intensive care units of hospitals in Egypt. International Nosocomial Infection Control Consortium(INICC) findings. 2012; 5:394-402
- Memish ZA, Stephens GM, Steffen R, Ahmed QA. Emergence of medicine for mass gatherings: Lessons from the Hajj. Lancet Infect Dis 2012;12:56-65.
- Worth LJ, McLaws ML. Is it possible to achieve a target of zero central line associated bloodstream infections? Curr Opin Infect Dis 2012;25:650-7.
- Rich KL, Reese SM, Bol KA, Gilmartin HM, Janosz T. Assessment of the quality of publicly reported central line-associated bloodstream infection data in Colorado, 2010. Am J Infect Control 2013;41:874-9.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.