Clinical and Laboratory Predictors of Articular Disorders Among HIV‑infected Patients Seen at Teaching Hospital Southeast Nigeria
- *Corresponding Author:
- Dr. Celestine Chibuzo Okwara
Department of Medicine, University of Nigeria Teaching Hospital, P.M.B 01129, Enugu, Nigeria.
E-mail: okwaracc@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: HIV infection may be associated with different arthropathies that are often underdiagnosed. There is also paucity of reported studies of relationship between clinical and laboratory features of HIV‑infected patients and articular disorders. Aims: To determine the predictors of articular disorders among HIV‑infected patients seen at tertiary hospital Nigeria. Subjects and Methods: Hospital‑based cross‑sectional descriptive study. Subjects were recruited from outpatient clinics of the study centers. Persons aged 16 years and above were recruited via stratified sampling method. Subjects with trauma, degenerative arthritis, malignancy, hepatitis B surface antigen and anti‑hepatitis C virus positivity or previously known to have pulmonary tuberculosis or rheumatological disorders were excluded. Pretest‑improved semi‑structured questionnaire was administered to the recruited 480 subjects comprising 240 HIV positive subjects (HPS) and 240 HIV‑negative subjects (HNS). Blood for relevant laboratory tests and radiographs were done where necessary. Diagnosis of articular disorder was based on American College of Rheumatology and European Spondyloarthropathy Study Group classification guidelines. Statistical Package for Social Sciences version 15 (SPSS Inc., Chicago, IL, USA) was used for data entry, validation, and analysis. Results: Of the 480 participants, both HPS and HNS were made up of 95 males and 145 females. There was statistically significant difference between the frequency of occurrence of articular disorders among the HPS of 37.1% (89/240) and the HIV‑negative controls of 16.2% (39/240) (ï£2 = 26.63 P = <0.01). Arthralgia frequency of 29.6% (71/240), HIV‑associated arthritis 4.6%, (11/240) (Reiter’s disease 1.3% (3/240), undifferentiated spondyloarthropathy 1.3%, (3/240) and gout 0.4% (1/240) (were seen among the HPS. Only arthralgia was found among HNS. Erythrocyte sedimentation rate (ESR) and age were the best predictors of arthralgia presence. CD4+ T‑cell count was predictive of HIV‑associated arthritis. Conclusions: Articular disorders are commoner among HIV patients than HNS. ESR and age were the best predictors of Arthralgia presence among HIV‑infected patients. CD4+ T‑cell count was predictive of HIV‑associated arthritis.
Keywords
Articular disorders, HIV, Predictors, Southeast Nigeria
Introduction
HIV infection may be associated with different arthropathies that are often underdiagnosed.[1-8] These articular conditions (arthralgia, arthritis, and spondyloarthropathy) are either caused by the HIV infection itself, antiretroviral therapy, adaptive changes in the immune system or are secondary to microbial infections. There is paucity of reported studies of articular disorders among HIV-positive subjects (HPS) in Nigeria. There is also paucity of reported studies of relationship between clinical and laboratory features of HIV-infected patients and articular disorders. This study was embarked on to determine the predictors of articular disorders among HIV-infected patients seen at a tertiary institution in South East Nigeria.
Hypothesis
The erythrocyte sedimentation rate (ESR), duration of highly active antiretroviral therapy (HAART), age, CD4+ T-cell count, and viral load of the HPS are predictors of articular disorders among HPS.
Subjects and Methods
The study was a hospital based cross-sectional descriptive study. It was conducted at a Tertiary Health Institution in South East Nigeria. The HPS were recruited from the GOPD and special HIV clinics of the study center, and the controls from the Voluntary Confidential Counseling and Testing Centers. The recruitment of subjects for the study took place from August 3, 2011 to May 28, 2012. The study population was largely Igbo tribe. Ethical approval was obtained from the Ethics and Research Committee of the Tertiary Health Institution. Also, permission to use the HIV laboratory services and data repository was sought and obtained from the Harvard School of Public Health (President’s Emergency Plan for AIDS Relief) Boston USA (The sponsor of the HIV/ ARV Clinic). The provisions of Helsinki Declaration were observed.
The sample size (n) was determined using the WHO formula [9] for calculating the minimum sample size from an infinite population and prevalence rate of articular disorders among HIV patients in Kenya of 17%.[10] Thus n = 216 rounding up by 10%, for higher accuracy appropriate sample size = 240 HPS. The control was: 240 HIV-negative subjects (HNS). These gave a total of 480 subjects for the study.
Subjects were recruited by systematic random sampling of all the HPS attending the clinics of the tertiary health institution hospitals. Every third HPS attending the clinics was recruited if he or she was eligible and gave consent. Those that were not eligible or declined consent were dropped and the systematic sampling continued until the sample size was completed. The HPS was matched gender and age group wise with the control. A pretest questionnaire was administered to 30 HIV-positive and 30 HIV-negative subjects who were recruited randomly from the eligible participants to assess performance and applicability of the semi-structured questionnaire for detection of articular disorders. Observations made were used to improve on the semi-structured questionnaire, but the subjects for the pretest were however excluded from the main study.
The pretest-improved semi-structured researcher administered questionnaire was applied to the study population. It was used to assess the symptoms and the signs of articular disorders and tests results. The researcher administered questionnaire had sections containing examination based on the gait, arms, spine, and legs (GALS) locomotor screen. Those with abnormal GALS screen were further interviewed and examined as per the American College of Rheumatology (ACR) ad hoc committee on clinical guidelines for the initial evaluation of adults with acute musculoskeletal symptoms.[11]
Blood samples were drawn for ESR, viral loads and CD4+ T-lymphocyte cell count determination. HIV positivity was confirmed either by double ELISA (strip and micro-pit) or ELISA and Western blot. Patients with inflammatory arthritis additionally did serum uric acid, rheumatoid factor (RF) and antinuclear antibody tests (ANA). RF was done by latex agglutination test, and ANA tests were done by ELISA method. Standard digital radiographs of affected joints were taken in indicated subjects. Patients with hip and alternating buttock pains had plain radiography of both hips and sacroiliac joints (anteroposterior view) which were evaluated by same radiologist for features of avascular necrosis (AVN) and sacroiliitis, respectively. Synovial fluid was obtained, for analysis and microscopy, culture/sensitivity testing and acid fast bacilli detection in those with demonstrable joint effusion. The clinically evident articular features, laboratory, and radiographic findings were used to reach diagnosis of articular disorder. The diagnosis and classification for rheumatic articular disorders were based on ACR classification guidelines for these articular disorders[4,12,13] but on European Spondyloarthropathy Study Group (ESSG) for spondyloarthropathy.[14]
Statistical Package for Social Sciences 15 version (SPSS Inc., Chicago, IL, USA) was used for data entry, validation, and analysis.
Results
A total of 480 participants were recruited for the study comprising 240 HPS as test subjects and 240 HNS as control subjects. Both HPS and HNS were made up of 95 males and 145 females. The mean age of the HPS was 38.1 (10.2) years and ranged from 17 to 61 years. The controls had a mean age of 38.4 (10.5) years and ranged from 16 to 63 years. As expected, there was no statistically significant difference between the mean age of the HPS 38.1 (10.2) years and control 38.4 (10.5) years (t = 0.305, P = 0.76). Table 1a and b shows the age group distribution and the sociodemographics of the study population respectively.
Occupational distribution and marital status of respondents
They are shown in Table 2. Across all articular disorder groups, artisans were the most frequently afflicted occupational group.
Viral load, CD4+ T-cell count and erythrocyte sedimentation rate of the study population
The mean viral load of the 240 HPS was 133,697.36 (315,732.5) copies/µl. The median viral load was 5488.5 copies/µl. The Mann–Whitney U-test showed that the ranked mean (146.5) of the viral load of HIV-positive HAART naïve subjects was significantly higher than that (94.50) of HIV-positive HAART exposed subjects (U = 4080.5, P = <0.01).
The mean CD4+ T-lymphocyte cell count of HPS and controls were respectively 326.57 (240.7) cells/µl and 951.35 (332.3)/µl, while the median CD4+ T-lymphocyte cell count of HPS and controls were 234.0/µl and 864.0/µl, respectively.
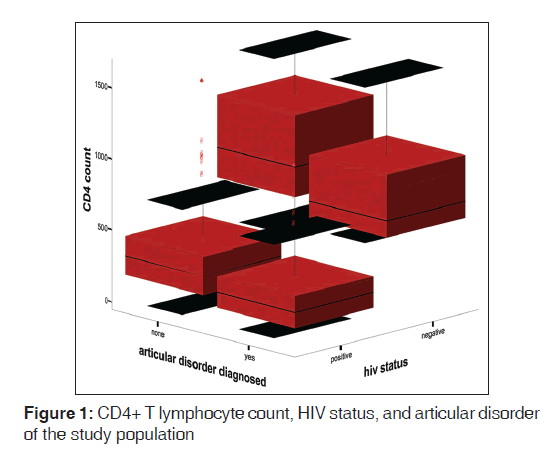
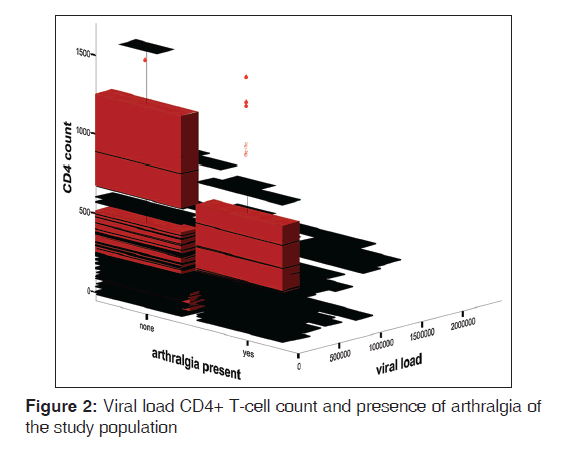
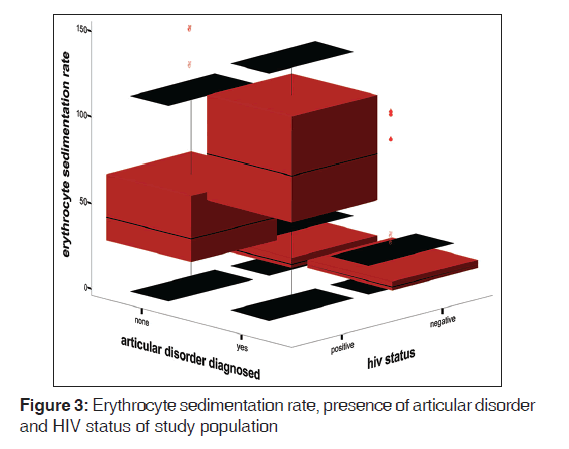
The mean ESR of HPS was 67.0 (42.4) mm 1st h while that of HNS was 15.0 (10.7) mm 1st h. There was statistically significant difference between the mean ESR of the HPS and HNS (t = 18.41, P < 0.01). Figure 1 shows the box plot graph of CD4+ T-cell count, articular disorder, and HIV status of the study population. From the Figure 2, it is clear that the viral loads for both HPS with arthralgia and without arthralgia were similar while the CD4+ T-cell counts were higher for HPS without articular disorders than the HPS with articular disorders. Figure 2 shows viral load, Arthralgia, and CD4+ T-cell counts of HIV-infected subjects. Figure 3 shows box plot of ESR, articular disorder, and HIV status of the study population.
Highly active antiretroviral therapy and study population
One hundred and twenty of the 240 HPS (i.e., half) were on HAART
Articular disorder diagnosis among HIV positive cases and controls
The frequency of all articular disorders in the population of HPS was 37.1% (89/240) while that among the control was 16.2% (39/240). There was statistically significant difference between the frequency of occurrence of articular disorders among the HPS and the controls (X2 = 26.63 P= <0.01). The frequencies of the different articular disorders found among the HPS are as follows: Arthralgia (29.6%, 71/240), HIVAA (4.6%, 11/240), Reiter’s disease (1.3%, 3/240), undifferentiated spondyloarthropathy (1.3%, 3/240), and gout (0.4%, 1/240). Only arthralgia was found among HNS.
The characteristics of the subjects and that of the articular disorders (arthralgia and HIVAA) are shown in Tables 1-3.
| Agegroup | Study population N (%) | TotalN (%) | ||||
|---|---|---|---|---|---|---|
| Test | Control | |||||
| 10-19 | 7 | (2.9) | 7 | (2.9) | 14 | (2.9) |
| 20-29 | 37 | (15.4) | 37 | (15.4) | 74 (15.4) | |
| 30-39 | 98 | (40.8) | 98 | (40.8) | 196 | (40.8) |
| 40-49 | 58 | (24.2) | 58 | (24.2) | 116 | (24.2) |
| 50-59 | 34 | (14.2) | 34 | (14.2) | 68 (14.2) | |
| 60-69 | 6 | (2.5) | 6 | (2.5) | 12 | (2.5) |
| Total | 240 (100.0) | 240 (100.0) | 480 (100.0) | |||
Table 1a: Age group distribution of study population
| Variable | HIV+ | HIV- | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Occupational distribution | |||||
| Unemployed | 15 | 6.3 | 9 | 3.8 | |
| Artisans | 81 | 33.8 | 77 | 32.1 | |
| Student | 16 | 6.7 | 23 | 9.6 | |
| Professional | 36 | 15 | 33 | 13.6 | |
| Trader | 67 | 27.8 | 71 | 29.6 | |
| Farmer | 25 | 10.4 | 27 | 11.3 | |
| Marital status | |||||
| Single | 177 | 73.8 | 158 | 65.8 | |
| Married | 57 | 23.8 | 60 | 25.0 | |
| Divorced | 6 | 2.5 | |||
| Co-habiting | 0 | 0 | 11 | 4.6 | |
| Separated | 0 | 0 | 10 | 4.2 | |
| Widow (er) | 0 | 0 | 1 | 0.4 | |
HIV: Human Immunodeficiency Virus
Table 1b: The socio-demographic features of the study population
| Variable | HIV positive | N | Percent | HIV negative | N | Percent | |
|---|---|---|---|---|---|---|---|
| Age group | |||||||
| 10-19 | 1 | 1.4 | 0 | 0 | |||
| 20-29 | 8 | 11.3 | 4 | 10.3 | |||
| 30-39 | 30 | 42.3 | 10 | 25.6 | |||
| 40-49 | 18 | 25.4 | 13 | 33.3 | |||
| 50-59 | 11 | 15.5 | 10 | 25.6 | |||
| 60-69 | 3 | 4.2 | 2 | 5.1 | |||
| Marital status | |||||||
| Single | 56 | 78.9 | 23 | 59 | |||
| Married | 10 | 14.1 | 10 | 25.6 | |||
| Divorced | 5 | 7.0 | 0 | 0 | |||
| Co-habiting | 0 | 0 | 1 | 2.6 | |||
| Separated | 0 | 0 | 4 | 10.3 | |||
| Widow(er) | 0 | 0 | 1 | 2.6 | |||
| Occupation | |||||||
| Unemployed | 2 | 2.8 | 0 | 0 | |||
| Artisan | 23 | 32.4 | 13 | 33.3 | |||
| Student | 2 | 2.8 | 2 | 5.1 | |||
| Professional | 16 | 22.5 | 6 | 15.4 | |||
| Trader | 16 | 22.5 | 7 | 17.9 | |||
| Farmer | 12 | 16.9 | 11 | 28.2 | |||
| HAART status | |||||||
| Naïve | 36 | 50.7 | |||||
| Exposed | 35 | 49.3 | |||||
| HAART regimens | |||||||
| None | 36 | 50.7 | |||||
| Tenofovir, emtricitabine and Nevirapine | 4 | 5.6 | |||||
| Tenofovir, emtricitabine and Efavirenz | 4 | 5.6 | |||||
| Efavirenz, lamivudine and Abacavir | 1 | 1.4 | |||||
| Zidovuldine, lamivudine and Nevirapine | 26 | 36. | |||||
HIV: Human Immunodeficiency Virus
Table 2a: The characteristics of HIV infected subjects with Arthralgia and HIV negative subjects with Arthralgia
| Variable | N | Percent |
|---|---|---|
| Complaint at HIV screening | ||
| Others | 51 | 71.8 |
| Joint pain + others | 17 | 23.9 |
| Joint pain | 2 | 2.8 |
| Asymptomatic | 1 | 1.4 |
| Joint count | ||
| Monoarticular | 3 | 4.2 |
| Oligoarticular | 24 | 33.8 |
| Polyarticular | 44 | 62.0 |
| Joint count | ||
| Fingers | 11 | 4.8 |
| Shoulder | 16 | 7.0 |
| Hip | 20 | 8.7 |
| Wrist | 28 | 12.2 |
| Elbow | 37 | 16.0 |
| Knee | 58 | 25.2 |
| Ankle | 60 | 26.1 |
| Total | 230 | 100.0 |
| Pain severity | ||
| Mild | 29 | 40.8 |
| Moderate | 41 | 57.7 |
| Severe | 1 | 1 |
| Functional status | ||
| Class I | 9 | 12.7 |
| Class II | 58 | 81.7 |
| Class III | 4 | 5.6 |
| Class IV | 0 | 0 |
| WHO HIV disease stage | ||
| State 1 | 1 | 1.4 |
| State 2 | 60 | 84.5 |
| State 3 | 10 | 14.1 |
HIV: Human Immunodeficiency Virus
Table 2b: Characteristics of HIV positive subjects with Arthralgia
| Variable | N | Percent |
|---|---|---|
| Age group | ||
| 30-39 | 6 | 54.5 |
| 40-49 | 4 | 36.4 |
| 60-69 | 1 | 9.1 |
| Gender | ||
| Male | 8 | 72.7 |
| Female | 3 | 27.3 |
| Occupation | ||
| Artisan | 4 | 36.4 |
| Trader | 4 | 36.4 |
| Farmer | 3 | 27.2 |
| Presenting complaint at HIV screening test | ||
| Arthritis | 1 | 9.1 |
| Others | 7 | 63.6 |
| Joint pain and others | 3 | 27.3 |
| HAART status | ||
| Naïve | 6 | 54.5 |
| Exposed | 5 | 45.5 |
| Joint count classification | ||
| Oligoarticular | 8 | 72.7 |
| Polyarticular | 3 | 27.3 |
| Pain severity | ||
| Moderate | 9 | 81.8 |
| Severe | 2 | 18.2 |
| Functional status | ||
| Class 2 | 7 | 63.6 |
| Class 3 | 4 | 36.4 |
| WHO HIV disease stage | ||
| Stage 2 | 3 | 27.3 |
| Stage 3 | 8 | 72.7 |
HIV: Human Immunodeficiency Virus
Table 3: Characteristics of subjects with HIV Associated Arthritis
Predicators of arthralgia, HIV-associated arthritis, spondyloarthropathy and Reiter’s disease among HIV-positive subjects
The backward logistic regression analysis of the hypothesized factors that predict arthralgia among HPS was done. The ESR, duration of HAART, age, CD4+ T-cell count, and viral load of the HPS were entered. ESR and age were the best predictors of arthralgia presence (OR = 1.018, P= <0.01 for ESR) and (OR = 1.035, P= 0.024 for age). For every 2-fold increase in ESR, arthralgia is 101 times more likely to be present than absent. For every 3-fold increase in age, arthralgia is 103 times more likely to be present than absent. Again looking at the contribution of each of the two variables with the other kept constant, 27.64% of the explained arthralgia variance was by ESR while only 5.25% by age. The overall specificity of this prediction was 71.7% that is quite good.
The logistic regression analysis of hypothesized factors that predict HIVAA among HPS was done with CD4+ T-cell count, ESR, age, viral loads, and HIV disease duration entered into the equation initially. The result shows that only CD4+ T-cell count was predictive of HIVAA (OR = 0.994, P= 0.01). For every 6-fold decrease in CD4 T-cell count, HIVAA is 994 times more likely to be present than absent.
The logistic regression analysis of hypothesized factors that predict undifferentiated spondyloarthropathy, Reiter’s disease among HPS was done with CD4+ T-cell count, ESR, Age, viral loads, and HIV disease duration entered into the equation initially. The result shows that none was predictive of undifferentiated spondyloarthropathy, Reiter’s disease (P > 0.05 in all).
Discussion
Articular disorders are frequent but often under-diagnosed manifestation in patients infected with HIV.[1-8] The prevalence of articular disorders in HPS has been reported to be 4.6–70%.[4-8,10] The reported range is wide and reflects prevalence from different regions of the globe and can be explained by differences in definitions, in populations characteristics, and in environments. In Nigeria, Adelewo et al. reported five cases to illustrate the spectrum of rheumatological manifestations of HIV among Nigerians.[15] In other parts of Africa and globally, available reports are on the rate of positivity to HIV antibodies among patients with certain articular disorders.[16,17] This study has added further statistics to the available studies and case reports on articular disorders among HIV-infected persons.[5,6,8,10,13-18] The prevalence of articular disorders in HPS of 37.1% found in this study is in keeping with previous reports of 4.6–70%.[2,4-8,10,13] The significantly higher prevalence compared to controls suggested that HIV infection may be associated with articular disorders. The prevalence got in this study may be close to the true value because the subjects recruited for this prospective study were evaluated and classified based on ESSG and ACR classification guidelines for these articular disorders. Again viral infection like hepatitis viruses C and B were excluded during recruitment of subjects.
The similarity of the overall occupational distributions of the HPS and controls reflect the occupational distribution of residents of Enugu. Across all articular disorder groups, Artisans were the most frequently afflicted occupational group. This is worrisome because being artisans they needed their joints to be functionally optional. The increased joint problem among these artisans with HIV infection predisposes them to functional impairment and decreased productivity thereby deepening the burden that HIV infections had put on them.
The articular disorder types among HIV-positive and HIV-negative subjects
The articular disorders reported among HPS include Reiter’s syndrome, HIV-associated arthritis, painful articular syndrome, acute symmetric polyarthritis, undifferentiated spondyloarthropathy, AVN of bone heads, septic arthritis, arthralgia, psoriatic arthritis, and gout. The control population also have articular disorders reportedly including any form of articular disorder including inflammatory, infective, degenerative, and neoplastic articular disorders.
The design of this study excluded the articular disorders that are degenerative, neoplastic or related to medications other than HAART. Thus persons with osteoarthritis, tuberculostatic medications, medications with hyperuricemia adverse effects, malignancies were not recruited as subjects.
The HPS in this study was found to have only arthralgia, HIV-associated arthritis, Reiter’s disease, gout and undifferentiated spondyloarthropathy. None had painful articular syndrome, acute symmetric polyarthritis, AVN of head of bone, septic arthritis, and psoriatic arthritis.
The absence of painful articular syndrome and acute symmetric polyarthritis may be explained by the fact that their prevalence was very low among the HPS in Enugu to be picked in the course of the cross-sectional study or that the HPS in the recruitment area Enugu do not have these articular disorder types. Their being acute and transient articular disorders may have made their prevalence to be very low and thus not found within the study population and period.
The absence of AVN of head bone and psoriatic arthritis may be a characteristic of HPS in the areas of recruitment. For the AVN, the recruited subjects had HIV infection, half were on HAART but median/mean CD4+ T-cell count were well above 60/µl. The HIV positivity and percentage of them on HAART were among the reported risk factors for AVN.[19] But CD4+ T-cell count less 60/µl was reported as a risk factor for development of AVN of head of bone.[19]
The absence of septic arthritis is interesting. Septic arthritis was thought to be expectedly high among HPS. However, overtime this has proved to be untrue. There has not been demonstration of any clear relationship between CD4 count and septic arthritis.[20] In fact, HIV predisposing to septic arthritis has been disputed.[20] It is rather increasingly thought to be related to simultaneous intravenous drug abuse. This era of increased HIV awareness, better health seeking behavior of HIV-infected subjects, early diagnosis, HAART and availability of more potent antibiotics may be the explanation for the absence of septic arthritis among the HPS studied. Moreover, intravenous drug abuse is not common in Enugu Nigeria.
For the HNS, the articular disorder found among them was arthralgia. Exclusion criteria eliminated most of the other articular disorders (osteoarthritis, malignant arthropathies, trauma, etc.) that could be seen among the subjects.
Predictors of articular disorders among HIV subjects
The ESR and age of HPS were both found to be predictive of Arthralgia. Every 2-fold increase in ESR value was predictive of 101 times likelihood of arthralgia presence than arthralgia absence while increase 3-fold in age was required for 103 likelihood of arthralgia presence than absence suggesting that ESR of the subjects was a better predictor of arthralgia presence than age. ESR is a marker of systemic inflammation, hence higher ESR values, indicating widespread systemic inflammation may explain the significantly higher frequency of arthralgia with higher ESR values. It also explains partly the predictability of arthralgia incidence with higher ESR. For the age, older age goes with same immune dysregulation which is worse in the HIV-infected persons. The contribution of age to prediction as shown in the result is small; however the immune dysregulation and the wear and tear that goes with aging may be the explanation for increasing frequency/predictability of arthralgia with advancing age.
The logistic regression strongly suggested that ESR, age, viral loads, and CD4+ T-lymphocyte count have no relationship with Reiter’s disease and undifferentiated spondyloarthropathy among the HPS studied. Reiter’s disease has been reported in HIV disease among young sexually active persons. It is thought to be strongly linked to the sexually transmitted disease presence in the HIV-infected persons. It is an autoimmune inflammatory process directed against the joint elements mimicking components of infective sexually transmitted agents. Thus age, viral loads, CD4+ T-lymphocyte count may not be predictive of Reiter’s syndrome and undifferentiated spondyloarthropathy as seen in this study.
Logistic regression analysis was done for the HIV patients with HIVAA. Of the five continuous variables (VL, CD4+ T-lymphocyte count, age, ESR, HIV disease duration) entered initially, only CD4+ T-lymphocyte count was found to be predictive of HIV-associated arthritis.
Strengths and limitations
We could not do all possible tests to exclude possible confounding etiologies of articular disorders. So there was still a possibility of misclassifications. There is also a possibility that our HIV patients presented with asymptomatic exclusion criteria (e.g., HIV with malignancy) then causing confusion. However, because we matched our test subjects and controls, drew the two samples also from same recruitment area, in same geographical and had same exclusions and inclusions criteria for both sub-populations, there is no reason why this possibility of misclassification would affect our results.
Conclusion
The study showed that articular disorders are commoner among HPS than HNS. HIV infection may have a significant effect on articular disorder development. Arthralgia was the most frequent articular disorder among both HPS and HNS. ESR and age were the best predictors of Arthralgia presence among HIV-infected patients. CD4+ T-cell count was predictive of HIV-associated arthritis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Anthony S, Fauci H, Clifford L. Human Immunodeficiency Virus (HIV) disease: AIDS and related disorders. Immunodeficiency virus disease and AIDS. In: Dennis LK, Eugene B, Anthony SF, Stephen LH, Dan LL, Jameson JL, editors. Harrison’s Principles of Internal Medicine. 17th ed., Vol. 309. New York: McGraw-Hill; 2008. p. 1852-910.
- Luzzi GA, Peto TE, Weiss RA, Conlon CP. Human immunodeficiency virus and acquired immunodeficiency syndrome. In: Warrell DA, Cox TM, Firth JD, Benz EJ, editors. Oxford Textbook of Medicine. 4th ed., Vol. 1, Sec. 7.10.21. Oxford; United Kingdom: Oxford University Press; 2003. p. 422-42.
- Wilkins EG. Human immunodeficiency virus infection and the human acquired immunodeficiency syndrome. In: Nicholas AB, Nicki RC, Brian RW, editors. Davidson’s Principles and Practice of Medicine. 20th ed., Vol. 14. Edinburgh: Elselvier; 2006. p. 377-402.
- Mody GM, Parke FA, Reveille JD. Articular manifestations of human immunodeficiency virus infection. Best Pract Res Clin Rheumatol 2003;17:265-87.
- Medina-Rodriguez F, Guzman C, Jara LJ, Hermida C, Alboukrek D, Cervera H, et al. Rheumatic manifestations in human immunodeficiency virus positive and negative individuals: A study of 2 populations with similar risk factors. J Rheumatol 1993;20:1880-4.
- Berman A, Espinoza LR, Diaz JD, Aguilar JL, Rolando T, Vasey FB, et al. Rheumatic manifestations of human immunodeficiency virus infection. Am J Med 1988;85:59-64.
- Rynes RI, Goldenberg DL, DiGiacomo R, Olson R, Hussain M, Veazey J. Acquired immunodeficiency syndrome-associated arthritis. Am J Med 1988;84:810-6.
- Muñoz Fernández S, Cardenal A, Balsa A, Quiralte J, del Arco A, Peña JM, et al. Rheumatic manifestations in 556 patients with human immunodeficiency virus infection. Semin Arthritis Rheum 1991;21:30-9.
- Araoye MO. Research methodology with statistics for health and social science. 1st ed. Ilorin: Nathadox Publishers; 2004. p. 115-20.
- Ekwom PE. Prevalence and Characteristics of Articular Manifestations in Human Immunodeficiency Virus Infection as Seen at Kenyatta National Hospital, Nairobi, Kenya. Presentation at ACR/ARHP. Annual Scientific Meeting. Abstract; online EPUB; 2009.
- Vasquez A. Musculoskeletal disorders and iron overload disease: Comment on the American college of rheumatology guidelines for the initial evaluation of the adult patient with acute musculoskeletal symptoms. Arthritis Rheum 1996;39:1-8.
- Willkens RF, Arnett FC, Bitter T, Calin A, Fisher L, Ford DK, et al. Reiter’s syndrome. Evaluation of preliminary criteria for definite disease. Arthritis Rheum 1981;24:844-9.
- Mody G, Neeta P. Articular syndromes and HIV; CME. SA J CPD 2011;29:318-21.
- Gomariz EM, del M, Guijo VP, Contreras AE, Villanueva M, Estévez EC. The potential of ESSG spondyloarthropathy classification criteria as a diagnostic aid in rheumatological practice. J Rheumatol 2002;29:326-30.
- Adelewo OO, Oguntona S, Kolapo KO. Rheumatological manifestations of HIV infections among Nigerians: Case reports and literature review. Niger Med Pract 2005;47:117-21.
- Mijiyawa M, Oniankitan O, Khan MA. Spondyloarthropathies in sub-Saharan Africa. Curr Opin Rheumatol 2000;12:281-6.
- Njobvu PD, McGill PE. Is HIV the culprit? Spondyloarthropathy in the third world. J Rheumatol 1999;26:2074-5.
- Blanche P, Saraux A, Taelman H, Sicard D, Menkes CJ. Arthritis in HIV infection. A prospective study of 76 cases in Rwanda. Presse Med 1993;22:1128-32.
- Mary-Krause M, Billaud E, Poizot-Martin I, Simon A, Dhiver C, Dupont C, et al. Risk factors for osteonecrosis in HIV-infected patients: Impact of treatment with combination antiretroviral therapy. AIDS 2006;20:1627-35.
- Saraux A, Taelman H, Blanche P, Batungwanayo J, Clerinx J, Kagame A, et al. HIV infection as a risk factor for septic arthritis. Br J Rheumatol 1997;36:333-7.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.