Clinical Diagnosis of Noonan Syndrome and Brief Review of Literature
2 National Research Institute on Health Sciences, Brazzaville, Congo, Email: henriettepoaty@gmail.com
3 Department of Paediatrics, University Hospital Center, Brazzaville, Congo
Citation: Poaty H, et al. Clinical Diagnosis of Noonan Syndrome and Brief Review of Literature. Ann Med Health Sci Res. 2017; 7: 76-79
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
We report a six-year-old female child with clinical features evocative of the Noonan syndrome: short stature, short neck associated with distinct facial dysmorphism and congenital heart defects, skeletal anomalies and excessive hair growth on the anterior trunk. She has no mental retardation. Chest X-ray revealed cardiomegaly and echocardiography, a pulmonary stenosis with atrial septal defect. Cytogenetic analysis identified 46, XX, eliminating a Turner syndrome.
Keywords
Noonan syndrome; Genetic disorder
Introduction
Noonan syndrome (NS), also called “female pseudo-turner syndrome”, or “Turner phenotype with normal karyotype” is a common genetic disorder (OMIM # 163950). It has been described by Jacqueline Anne Noonan and Dorothy Ehmke in 1963 and its incidence range lies between 1:1,000 to 1:2,500 live births, affecting equally both males and females. [1-3]
Phenotypically, the disease is characterized by a short stature in 93% of cases, a typical triangular craniofacial dysmorphism combining wide spaced eyes, a broad forehead, down-slanting palpebral fissures, low set ears posteriorly rotated, and a short and webbed neck. [4,5] Another characteristic clinical feature is the constant presence of congenital heart defects in up to 90% of cases (OMIM # 163950), from which the most common type is pulmonary stenosis (mainly pulmonary valve stenosis in 50-65% of cases) associated to hypertrophic cardiomyopathy (in 20%). [3] The phenotype often includes skeletal deformities (extremities, chest or spinal anomalies), ophthalmic problems in 90% of cases (strabismus, nystagmus, cataract), cryptorchidism in 80% of male cases, renal malformations, oral anomalies (high arched palate, malocclusion, impacted teeth, retroclined mandibular incisors), coagulation defects, hypogonadism. Approximately 10% to 25% of the patients have hearing difficulties. [1,4,6] The most affected persons have learning capacities, and mental retardation is observed only in 25% of affected individuals. [6]
Worldwide congenital malformations are increasing, especially in mineral and petroleum producing African countries. [7-9] Thus, it is important to have a good knowledge of the most common congenital diseases, particularly those of genetic origins. The goal of the present paper is to improve from one case report the clinician’s knowledges on clinical diagnosis about a rare type of congenital disease, the Noonan syndrome.
Patients and Observations
The subject was a six-year-old female child of Congolese origin. The reason of consultation was a short stature and a weight delay. The mother was 25 years-old and the father was 32 years-old, and none of them presented particular familial history and no notion of parental consanguinity was found. The child was the first born in the family, from a normal term pregnancy. Prenatal ultrasound screening of second trimester was no significant. Birth weight was normal. At two months, the cardiac auscultation revealed presence of a systolic murmur.
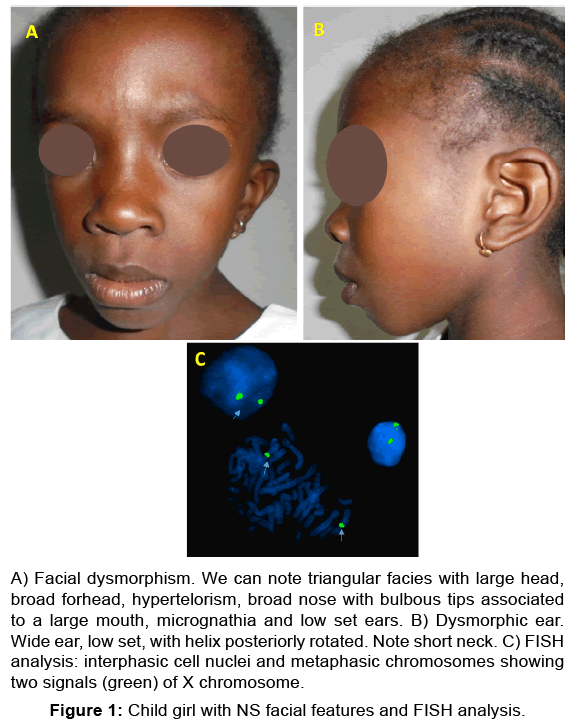
She presented short stature (size, 88 cm and weight, 12 kg). We noted a facial dysmorphism associating a triangular facies with hypertelorism [Figure 1A], wide forhead, proeminent eyes with antimongoloid slant, long philtrum, large low-set and posteriorly rotated ears with a thickened helix [Figure 1B]. Furthermore, she had excessive hair growth on anterior trunk, short neck, skeletal anomalies including chest deformity (broad thorax), small hands and feet. She presented no abdominal mass and external genital organs were normal. No learning difficulties or mental retardation were noted. On the contrary the child was very awake.
Figure 1: Child girl with NS facial features and FISH analysis.
A) Facial dysmorphism. We can note triangular facies with large head,
broad forhead, hypertelorism, broad nose with bulbous tips associated
to a large mouth, micrognathia and low set ears. B) ysmorphic ear.
Wide ear, low set, with helix posteriorly rotated. Note short neck. C) FISH
analysis: interphasic cell nuclei and metaphasic chromosomes showing
two signals (green) of X chromosome.
Chest X-ray revealed cardiomegaly, and echocardiography showed a stenosis of pulmonary artery trunk and atrial septal defect. The cytogenetic analysis based on karyotype and fluorescence in situ hybridization (FISH) performed from cell lymphocytes identified 46, XX, which eliminated a case of Turner syndrome [Figure 1C].
Thus, the clinical findings suggest the presence of a Noonan syndrome supported by normal cytogenetic analysis.
Discussion
Clinical features of our patient are not different from those described in literature in other populations affected by NS, once they are similar to those found on Senegalese patients suffering from NS. [1] A recent comparative study using facial analysis technology on a large cohort of NS patients in diverse populations (African, Asian, Latin American and Caucasian) reports that the most constant signs are equally present: hypertelorism and lowset ears in 80% of the cases, short stature in more than 70% of the cases and pulmonary stenosis in 48-53% of patients. [10] All these clinical manifestations were present in our patient.
The facial recognition technology is a new tool in positive clinical diagnosis of NS. It is based on facial anthropometric measurements. It consists in recognizing Noonan facial features by scanning the child’s face and in measuring facial landmarks (height of forehead, physiognomical height of the upper face, intercanthal width, and width of face, vermillon height of the upper lower lip, width of mouth, and width of auricle and length of auricle). [10-12] Thereafter, the results are compared with faces in database (standard set of normal and abnormal values). The method is currently used in medicine, particularly in the clinical diagnosis of genetic disorders such as the Digeorge syndrome or the Noonan Syndrome. [10-12] For NS, the sensitivity of such technology is 94%. [10]
According to literature, cardiac malformations found in NS can be variable. Others cardiac defects described are: atrial septal defects noted in 20% of cases, tetralogy of Fallot, ventricular septal defect and persistent ductus arteriosus. [3,4,6] Furthermore, the affected persons are predisposed to malignant tumors, particularly brain tumors (neuroblastoma, astrocytoma, gliomastoma), haematological malignancies (such as acute lymphoblastic leukemia or acute myeloid leukemia) and rabdomyosarcoma. [6,13] Other cancers are sertoli cell testis tumors, melanoma, and colorectal cancer. [6,13]
The syndrome can be suspected in prenatal period by ultrasound screening. However, hydramnios, intrauterine growth retardation, cardiac defect, mainly type conotruncal, nuchal lucency, and cystic hygroma are not specific signs of the NS. [14]
In fact, the clinical recognition of the NS can induce problems at three levels. i) The clinical signs evolve with age, making diagnosis less easy in affected adults. ii) Clinical expressivity in NS is variable, sometimes with minor clinical anomalies. iii) Many various genetic syndromes exhibit the same clinical manifestations as NS. However, we can note that in the latter, facial features are coarse; and that cardiac defects, dermatologic anomalies, hair anomalies and mental retardation are more distinctive. [3] Briefly, we have in Table 1 compared the main distinctive features of NS with the resembling NS clinical conditions. [2-4,6,14-17]
| Diseases | Clinical features | Incidence (lb or NWC) | MI | Altered gene/locus or chromosome |
|---|---|---|---|---|
| Noonan syndrome [2-4,6,16] | Short stature, typical facial dysmorphy, cardiac defect (pulmonary artery stenosis ++), rare cognitive delay. |
1:1000 - 1:2500 lb |
AD | PTPN11 in 50%, SOS1, RAF1, NRAS, Kras, BRAF, CBL, SHOC2, MEK1 |
| Turner syndrome (ORPHA: 881) | Short stature, facial dysmorphy, cardiac defect (aortic coartaction ++), renal anomalies, some with low cognitive delay. | 1:2500 lb | Loss of X or Y | |
| Costello syndrome [4,6] (ORPHA: 3071) |
Short stature, coarse facial features (broad nasal bridge), pulmonary valvular stenosis, frequent arrhythmias ++, curly hair, more malignancies, mental retardation. | 300 NWC | ICAD? | HRAS in 85% (11p15.5) |
| Leopard syndrome [4,6] (OMIM #613707) |
Growth retardation, facial dysmorphy (hypertelorism ++), pulmonary valve stenosis, ECG anomalies ++, genital anomalies, deafness, cognitive deficits. | 200 NWC | AD | PTPN11(12q24.13), RAF1, BRAF (7q24) in 17% |
| Neurofibromatose type 1 [14] (OMIM #16220) |
Short stature, facial dysmorphy, multiple café-au-lait spots, lisch nodules, neurofibromas, skeletal lesions (scoliosis), learning disabilities. | 1:3000- 1:4000 lb |
AD | NF1 more than 90% (17q11.2) |
| Waston syndrome [4] | Short stature, macrocephaly, café-au-lait spots, neurofibromas, pulmonary valve stenosis, lisch nodules, dull intelligence. | U | AD | NF1 |
| Cardiofaciocutaneous syndrome [15] | Distinctive coarse facial features, heart defect (pulmonary stenosis, arrhythmias), cutaneous, ocular gastrointestinal, neurologic anomalies, mental retardation | 1/810 000 lb | AD | BRAF, MEK1, MEK2, KRAS |
| Baraitser-Winter syndrome [15,17] (OMIM #243310) |
Short stature, facial dysmorphy (hypertelorism, eye coloboma, ptosis, thin upper lip), aortic valve anomaly, cognitive delay. | U | AD | ACTB (7p22.1) ACTG1(17q25.3) |
| MI:Mode Of Inheritance; Lb: Live Birth; ECG: Electrocardiogram; IC: Isolated Cases; AD: Autosomal Dominant; NWC: Number Of Worldwide Cases; U:Unknown. | ||||
Table 1: Differential diagnosis of NS with others resembling NS conditions. [2-4,6,14-17]
Because of the variability of expression and the presence of Noonan-like syndromes, it is important to know the distinctive clinical features of NS, especially in context of African countries where molecular diagnosis is not always accessible.
Genetically, NS is an autosomal dominant condition caused by many mutated genes. In most cases (60% of patients), it is sporadic due to de novo mutation: meiotic error (in oocyte or sperm) or post zygotic error. [4,14] The risk of recidive is less than 1%. [4] It may also be inherited from one parent. In the latter case, the risk of recidive is 50%. [4]
Heterozygous mutations are identified in numerous genes. [3,6] PTPN11 (tyrosine phosphatase non-receptor type 11) gene mapped on 12q24.13 is the most concerned, with 45 to 50% of the subjects affected by NS. [1,6,13] This gene is involved in various cellular processes such as cell growth, differentiation, mitotic cycle, and oncogenic transformation (2013-GRCh38/ hg38). Other altered genes indexed as causative for NS are SOS1 (10% of cases) mapped on 2p22.1, RAF1 (5-15%) located on 3p25.2, NRAS and Kras gene (less than 2-5%), BRAF gene (less than 2%) present on 7q34, CBL (less than 1%) or SHOC2, MEK1 and MEK2 (2013-GRCh38/hg38). [6,14] All genes actually indexed in NS are involved in RAS-MAPK (mitogen activated protein kinase) signal transduction pathway. There exists a good genotype-phenotype correlation and the molecular analysis (PCR, DNA sequencing) confirms the diagnosis approximately in 70-75% of patients. [4,6,14]
Concerning medical care, currently it exists no specific curative treatment, but recombinant human growth hormone in doses up 0.066 mg/kg/day is proposed, allowing height gain of 10.9 cm for men and 9.2 cm for women. [6,14] Adult stature can be normal (as in general population) approximately in 50% of cases. [14] The cardiac defects can be treated surgically.
Conclusion
The present study focused on clinical manifestations of NS, and showed that the condition has distinctive clinical features, especially in its typical form. The wide spectrum of clinical expressivities of NS and Noonan-like phenotypes can cause confusion. However, the NS diagnosis can be based on clinic as in our patient. A good knowledge of clinical features and the use of facial analysis technology (that we wish, will be made available to physicians in developing countries) can help clinicians to identify Noonan syndrome.
Acknowledgements
We are grateful to Pr Jean Jacques Candelier for the critical manuscript.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Ndiaye R, Ndiaye C, Leye M. Mutation N308T of protein tyrosine phosphatase SHP-2 in two Senegalese patients with Noonan syndrome. J Med Genet Genomics. 2014; 6: 6-10.
- Essawi ML, Ismail MF, HAfifi H. Mutational analysis on the PTPN11 gene in Egyptian patients with Noonan syndrome. Journal of the Formosan Medical Association. 2013; 112: 707-712.
- Verloes A, Cave H. Le syndrome de Noonan. MédecineFœtaleetEchographie en Gynécologie. 2010; 84: 10-17.
- Bhambhani V, Muenke M. Noonan syndrome. Am Fam Physician 2014; 89: 37-43
- Van der Burgt I. Noonan syndrome. Orphanet J Rare Dis. 2007; 2: 4
- Roberts AE, Allanson JE, Tartaglia M. Noonan syndrome. Lancet. 2013; 381: 333-342.
- Poaty H, Gentien D, Reyes C. Macroscopic analysis of fetus having arhinencephaly, synophthalmia and holoprosencephaly. Human Genet Embryol. 2016; 6: 135.
- Berthe MLA, Alphonse M. Epidemiology of cardiovascular diseases in children at the teaching hospital of Brazzaville, Congo. World Journal of Cardiovascular Diseases. 2016; 6: 410-424.
- Otaigbe BE, Tabans PN. Congenital heart disease in the Niger delta region of Nigeria: a four-year prospective echocardiographic analysis. Cardiovasc J Afr. 2014; 25: 265-268.
- Kruszka P, Porras AR, Addissie YA. Noonan syndrome in diverse populations. Am J Med Genet A.2017; 173: 2323-2334.
- Brown SC, Henderson BD, Buys DA. Cardiac abnormalities and facial anthropometric measurements in children from the Free State and Northern Cape provinces of South Africa with chromosome 22q11.2 microdeletion. Cardiovasc J Afr. 2010; 21: 32-36.
- Kruszka P, Addissie YA, McGinn DE. 22q11.2 deletion syndrome in diverse population. Am J Med Genet. 2017; 173: 879-888.
- Mutesa L, Pierquin G, Janin N. Germline PTPN11 missense mutation in a case of Noonan syndrome associated with mediastinal and retroperitoneal neuroblastictumors. Cancer Genet Cytogenet. 2008; 182: 40-42.
- Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best Pract Res ClinEndocrinolMetab. 2011; 25: 161-179.
- Pierpont ME, Magoulas PL, Adi S. Cardio-facio-cutaneous syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2014; 134: e1149-e1162.
- Mallineni SK, Yiu CK Y, King NM. Oral manifestations of Noonan syndrome: review of the literature and a report of four cases. Rom J MorpholEmbryol. 2014, 55: 1503-1509.
- Verloes A,Di Donato N,Masliah-Planchon J. Baraitser-Winter cerebrofrontofacial syndrome: delineation of the spectrum in 42 cases. Eur J Hum Genet. 2015; 23: 292-301.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.