COL4A2 BRAIN SMALL VESSEL DISEASE (A CASE REPORT OF PREVIOUSLY UNREPORTED MUTATION)
Received: 01-Sep-2022, Manuscript No. AMHSR-22-69977; Editor assigned: 05-Sep-2022, Pre QC No. AMHSR-22-69977; Reviewed: 16-Sep-2022 QC No. AMHSR-22-69977; Revised: 23-Sep-2022, Manuscript No. AMHSR-22-69977; Published: 30-Sep-2022
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
A 50 year old male patient presented with right sided weakness, dysarthria and ataxia. Patient’s detailed history was suggestive of 9/11 family members with history of premature death secondary to stroke. Neurological and Clinical Exome Sequencing (CES) utilizing next generation sequencing was used for genetic evaluation and variables. The results suggested COL4A2 variant OMIM phenotype brain small vessel disease with autosomal dominant inheritance. The patient under study having phenocopy of COL4A2 related mutation is described.
Keywords
Brain small vessel disease, COL4A2; Stroke; Exome sequencing; CADASIL
Introduction
The phenotypic spectrum of COL4A1/A2 related multi-spectrum disorders are rare but up-surging. Late onset, asymptomatic, sporadic and cases with de-novo mutations with multiple risk factors have been described [1].
Hereditary forms of subcortical small vessel disease, CADASIL, accounts for 50% of patients, followed by 13% of COL4A1/A2 mutations. COL4A1/A2 variants may be associated with coronary heart disease in the young. Often cases presents with polencephaly, scattered white matter lesions, carotid aneurysms, myopia, cerebellar and visual abnormalities [2,3]. The deletion of a variant in the COL4A1/A2 is partially consistent with phenotype features in the text patient. The results carefully are correlated with patient’s clinical profile and imaging studies.
Case Report
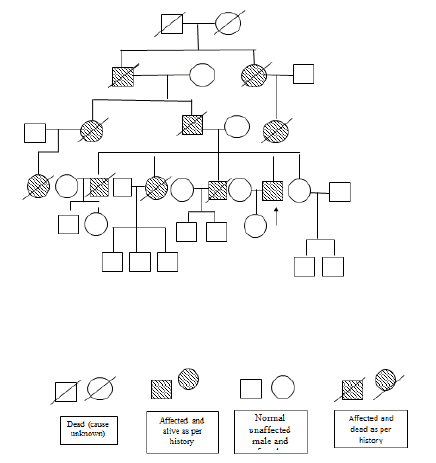
A 50 year old male presented with right upper and lower extremity weakness, progressive gait difficulties and dysarthria for 1 month. A week before the present hospitalization, he had a fall while climbing the stairs following dizziness. He denied history of concussion, vomiting, diplopia, headache or seizures. His past medical history was negative for migraine, hypertension, hyperlipidemia, diabetes, alcohol or tobacco use. Family history was noted significant for onset of ataxic symptoms in his father, grandfather and other extended family. Onset of symptoms was between ages 35 years-45 years with mortality in next 4.5 to 5 years. 9/11 family members died due to stroke. Details shown in pedigree of the patient. All affected members of the family had right sided weakness, swaying and slurred speech (Figure 1).
Patient’s neurologic examination was noted for dysarthria, unsteady wide gait and brisk deep reflexes in bilateral lower extremities. Cerebellar signs such as finger-nose test, abnormal heel-shin test and dysdiadokokinesia were more prominent on right side. Extraocular movements, cranial nerves, fundic examinations were normal. Nystagmus was not noted. All sensations were intact. Neuropsychiatric test were negative for cognitive impairment. Other systemic examinations were unrevealing.
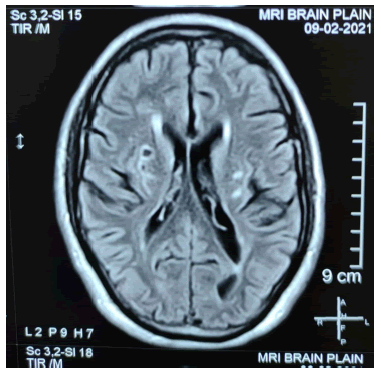
Extensive blood workup was unremarkable for thyroid function, lipid profile, sugar, HbA1C, homocysteine and sickling. Imaging included MRI brain which revealed area of restricted diffusion on DW images with corresponding low ADC values noted left centrum semiovale and pons on left side. Hyperintensities on T2 and FLAIR images suggestive of acute infarcts, with evidence of chronic infarcts noted in bilateral putamen and left half of pons. Findings also reveal mild cerebellar atrophy, dilated Virchow robin spaces bilaterally. MR angiography of the brain vessels did not reveal any significant abnormality. The fundus examination and fluorescence angiography were normal. Dermal biopsy appeared unremarkable and did not show evidence of Granular Osmiophilic Material (GOM) along the vessels. There was no evidence of congophilic amyloid deposits (Figure 2).
Base on patient’s history, medical evaluation, significant family history of similar illnesses with autosomal dominant affection, hereditary form of cortical and subcortical small vessel disease was entertained. He was suspected to have mutation in NOTCH3 gene and evaluated for genetic variations. Genetic study was undertaken at DNA lab India, with clinical exome sequencing utilizing next generation sequencing detected variant of COL4A2. The COL4A2 gene was 100% covered in the provided sample.
Discussion
Cerebral small vessel disease is an umbrella term which refers to intracranial vascular disease based on various clinicopathological processes and several clinical subtypes such as CADASIL, CARASIL, HDLS, CARASAL and COL4A disorders. The variants observed share both clinical and radiological features amongst them. Several genes and mutations have been identified in familial SVD [4].
CADASIL is one of the most common hereditary cerebral small vessel disease caused by mutation in NOTCH-3 gene. Mutations are localised from exon 2-24 which encodes EGFRs. Most of the mutations are missense type related to cysteine and residues. The disorder affects cerebral small vessels, brain white matter and deep gray matter. Presence of GOM is characteristic of the disease on dermal biopsy. Clinically and primarily CADASIL is characterized by migraine, stroke, mood disturbances and cognitive declines. Ataxia is an uncommon presentation with CADASIL.
In the present case all person who suffered had right sided weakness, swaying and slurred speech, a feature commonly noted in COL4A disorders. 9/11 affected members of the family had died of stroke with ataxia. All the unaffected members of the family are currently under 35 years of age. Symptoms often develop in affected person after age 45 and died within 3.5 years to 5.5 years within onset of symptoms. Because of financial limitation, gene mutation study could not be undertaken in unaffected family members.
Clinical Exome Sequencing [CES] undertakes selective capture and sequencing of the protein coding regions of the genes. Mutation are identified in exomic regions. Extracted DNA was used to perform targeted gene capture using a custom capture kit. The libraries were sequenced to mean-150x coverage data on sequencing platforms.
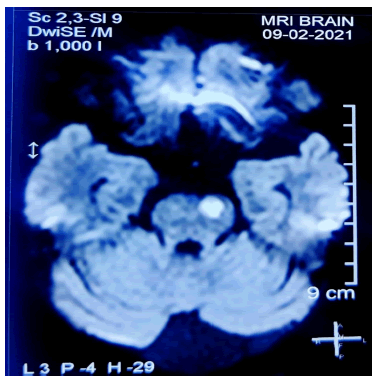
White matter lesion observed on MRI occur in specific area including corpus callosum and pons which are considered characteristic and as an important marker of small vessel disease. Dilated Virchow-Robin spaces seen on mri brain was previously found to be related to aging, hypertension in elderly person, now is considered an important marker of cerebral small vessel disease. It is due to its strong and independent association with white matter hyperintensities, lacunar infarcts and hereditary ischemic attacks (Figure 3).
In the present case presence of mega cisterna magna, dilated Virchow robin space bilaterally, infarcts in bilateral putamen, centrum semiovale and pons signifies sub cortical small vessel disease.
The clinical exome test of the patient was done from DNA lab India. The findings are summarized as below:
- OMIM phenotype brain small vessel disease-2 (OMIM#614483) is caused by a heterozygous nonsynonymous variation c.1837G>A in the exon 25 of COL4A2 gene was detected in the sample at depth of 87X. This variation causes a change of amino acid Glycine to Serine at position 613 in COL4A2 sequence [5].
- This variation has not been reported in any population data base.
- The COL4A2 gene is 100% covered in this sample.
- This variant c.1837G>A has been reported as VUS in the DNA lab India.
Since the variation has not been reported in literature, therefore based on the above evidence and considering ACFG classification guidelines, this COL4A2 gene has currently been classified as a variant of uncertain significance. The test results in the study are interpreted in the context of clinical findings, family history and lab data of the text case.
Limitations
Further testing of the unaffected family members are not carried out due to lack of financial aids. Sanger confirmation in the case is not carried out considering the clinical exome sequencing undertaken with NGS is done, a superior technology to Sanger.
Conclusion
Cerebral small vessels disease has been recognized as a important cause of cerebral dysfunctions with stroke and ataxia. The present case of cerebral SVD has been clinically and on genomic study correlated with COL4A2 variant of C1837 G>A , reported as variant of uncertain significance in the DNA lab India as per American college of genetics Where in COL4A2 gene was 100% covered in the sample. This variation has not been reported in the literature or any affected individual. Further significance of mutation can be assess only with time and availability of scientific evidences.
References
- Meuwissen ME, Halley DJ, Smit LS, Lequin MH, Cobben JM, Coo RD et al. The expanding phenotype of COL4A1 and COL4A2 mutations: Clinical data on 13 newly identified families and a review of the literature. Genet Med 2015; 17: 843-53.
[Crossref], [Google Scholar], [Indexed]
- Rannikmäe K, Davies G, Thomson PA, Bevan S, Devan WJ, FalconeGJ, et al. Common variation in COL4A1/COL4A2 is associated with sporadic cerebral small vessel disease. Neurology 2015;84:918-26.
[Crossref], [Google Scholar], [Indexed]
- Verbeek E, Meuwissen ME, Verheijen FW, Govaert PP, Licht DJ, Kuo DS, et al. COL4A2 mutation associated with familial porencephaly and small-vessel disease. Eur J Hum Genet. 2012;20:844–51.
[Crossref], [Google Scholar], [Indexed]
- Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman WB, Mancini GM, et al. COL4A2 Mutations Impair COL4A1 and COL4A2 Secretion and Cause Hemorrhagic Stroke. Am J Hum. Genet. 2012;90:91–101.
[Crossref], [Google Scholar], [Indexed]
- https;//www.omim.org/entery/614483




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.