Color Stability of Heat‑cure Acrylic Resin Subjected to Simulated Short‑term Immersion in Fast‑acting Denture Cleansers
- *Corresponding Author:
- Dr. Lohitha K
Flat No. 301, Savera Homes, 3/3, Vikas Nagar, Guntur - 522 006, Andhra Pradesh, India.
E-mail: kallurilohitha25@gmail.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Regular usage of denture cleansers is recommended in complete denture wearers for effective plaque control, and these cleansers alter the physical properties of acrylic resin over a period of time. Thus, an in vitro study was carried out to assess the effect of denture cleansers on the color stability of heat‑cure denture base resin. Aim: The aim of the present study was to evaluate the effect of commercially available fast‑acting denture cleansers on the color stability of heat‑cure denture base resin at different time intervals. Materials and Methods: Thirty‑six heat‑cure acrylic resin (Ivoclar Triplex Hot‑V) specimens are randomly allocated into four groups ‑ Group A (distilled water as control); Group B (polident ‑ 3 min); Group C (fixodent scope plus); and Group D (stain away plus) comprising of nine samples each. After recording the baseline values, the specimens were immersed in their respective cleansing solutions for a prescribed time interval. This procedure was repeated daily, and the color change (ΔE) was evaluated after 90 and 180 days interval using a colorimeter in a standard “Commission International de l’Eclairage” color system. Statistical Analysis Used: Paired t‑test and Dunnett’s T3 test. Results: All the groups exhibited a variable color change (ΔE) for an immersion period of 90 days. However, significant color differences (P = 0.001) were noticed among the test groups after 180 days. Conclusion: The color change of denture base resin was greater for Group D followed by Groups B, C, and A respectively after 180 days of immersion. The ΔE values of all test groups increased with time.
Keywords
Color stability, Colorimeter, Denture base resins, Denture cleansers
Introduction
The denture base is the part of a denture that rests on the foundation tissues and to which teeth are arranged.[1] Before 1940, various materials such as wood, bone, ivory, gold, porcelain, vulcanite, and celluloid, were used for the fabrication of denture base. However, none of these materials virtually represented the ideal denture base material.[2] From 1940 till date, acrylic resin is being used almost universally as a denture base material owing to their availability, dimensional stability, handling characteristics, color and compatibility with oral tissues.[3]
According to the International Organization for Standardization 1567, denture base polymers are classified into five groups as heat processing polymers (powder and liquid, plastic cake), autopolymerized polymers (powder and liquid, pour type resin), thermoplastic blank or powder, light-activated materials, and microwave-cured materials.[4] Apart from being used as denture base materials, acrylic polymers were also used as tray materials, denture teeth, provisional restorations, and in maxillofacial prosthesis fabrication.[5]
Color stability is an important physical factor demonstrating their serviceability and the aging or damage occurring to denture base resins over a period of time. Color change is due to various extrinsic and intrinsic factors affecting the physical and mechanical properties of acrylic resin over a period of time.[5,6] Effective denture plaque control is essential for complete denture wearers because plaque is a major factor in the etiology of denture stomatitis.
Denture cleansing may be performed by a number of products which are categorized briefly as mechanical and chemical cleansers. Mechanical procedures include brushing with a soap or an abrasive paste and water and are the most commonly used methods. However, effective biofilm removal requires a degree of manual dexterity that is often lacking particularly among elderly individuals. Thus, chemical cleansing, such as immersion in denture cleanser solutions is considered as a viable alternative.[7]
Commercial denture cleansers are categorized into the following groups as neutral peroxides with enzymes, acids, hypochlorites, peroxides, crude drugs, and mouth rinses. Thus, regular use of denture cleansers is advisable for effective plaque control.[8] However, daily usage of denture cleansers may affect the physical and mechanical properties of denture base materials.[6] Acrylic resin whitening is the main disadvantage of these denture cleansing solutions.[7]
Earlier denture cleanser formulations require overnight immersion for effective plaque control. Recently, newer formulations have been introduced which require only 3–10 min immersion for effective plaque control as recommended by the manufacturers. As the effect of recently introduced fast-acting denture cleanser formulations on the color stability of denture base resin material were not well documented in the dental literature, the present study was thus carried out to evaluate the effect of commonly used commercially available fast-acting denture cleansing agents on the color stability of heat-cure denture base material at different time intervals.
Materials and Methods
Before commencing this laboratory study, this study design was approved by the Institutional Ethical Committee.
Heat -cure acryl ic res in specimens measur ing 10 mm × 10 mm × 2 mm[1,7] were fabricated using a plastic mold in a conventional compression molding technique. Once the specimens were cured, they were retrieved, and finishing procedures were carried out using stone burs and 1200 grit abrasive waterproof paper. Later, polishing was done using pumice slurry and stored in distilled water for 24 h before testing to reduce the residual monomer content.
The prepared samples were then assessed for internal or external porosity, cracks or irregularities before randomly dividing them into the following groups, comprising nine samples each. Group A consists of distilled water as a control, Group B consists of polident - 3 min (GlaxoSmithKline, Consumer Healthcare), Group C consists of fixodent plus scope (Procter and Gamble), and Group D consists of Stain away plus (Regent Labs Inc.,) denture cleansing solutions. Before immersing the specimens in their respective cleansing solutions, baseline color values of all the samples were recorded. The test group specimens were immersed in their respective denture cleansing solutions as per the time interval recommended by the manufacturer (3 min immersion for Group B, 15 min immersion for Group C, and 10 min immersion for Group D) and the samples were thoroughly rinsed and immersed in distilled water at room temperature for the rest of the day. Control group specimens were rinsed and immersed in distilled water each day. The procedure was repeated each day subsequently for 180 days.
The color change of specimens in each group was evaluated at different time intervals (90 and 180 days, respectively) using a hunterlab colorflex colorimeter. The colorimeter was calibrated according to the manufacturer’s instructions before each measurement period using the white calibration plate supplied by the manufacturer. The color differences were evaluated using the CIE L*a*b* colorimetric system, which is based on three parameters for defining color: L*, a*, and b*. The color change (ΔE) of each specimen was calculated as follows:[9]
ΔE = ([ΔL*]² + [Δa*]² + [Δb*]²) 1/2
Where ΔL*, Δa*, Δb* represent the differences measured in L*, a*, and b* values between the baseline coordinates and those measured after immersion in different time intervals.
The levels of color change (ΔE) have been quantified by the National Bureau of Standards (NBS) with the NBS units of color difference. NBS units are expressed by the following formula:[10]
NBS unit = ΔE × 0.92
The whole procedure and collection of data are carried out by a single investigator. However, to avoid bias, a second investigator who is unaware of the prior results, evaluated the samples randomly. As the variation in the results was nonsignificant, the earlier values are only considered. The data thus obtained was tabulated and subjected to statistical evaluation.
Results
Mean and standard deviation was determined for all the groups tested and are tabulated in Table 1. The paired t-test was performed to determine whether there is any statistically significant difference of mean ΔE among time intervals (90 and 180 days) within three test groups. Comparison of mean ΔE among the groups for 90 and 180 days’ time interval is done using the ANOVA test. As the ANOVA value among the groups is highly significant for 180 days immersion interval, Dunnett’s T3 test has been applied to compare the significant color change between groups at 180 days immersion interval. All analyses were computed with statistical software (IBM SPSS Statistics 21.0.0.0 version).
| Groups | Time(days) | Mean?E(SD) | NBSunit |
|---|---|---|---|
| Control group (Group A) | 90 | 1.72 (0.86) | 1.58 |
| 180 | 2.25 (1.27) | 2.07 | |
| Polident - 3 min (Group B) | 90 | 1.6 (0.64) | 1.47 |
| 180 | 14.76 (1.04) | 13.57 | |
| Fixodent scope plus (Group C) | 90 | 1.68 (0.69) | 1.54 |
| 180 | 14.56 (1.28) | 13.39 | |
| Stainaway plus (Group D) | 90 | 1.65 (0.84) | 1.518 |
| 180 | 15.52 (1.07) | 14.27 |
SD: Standard deviation, NBS: National Bureau of Standards
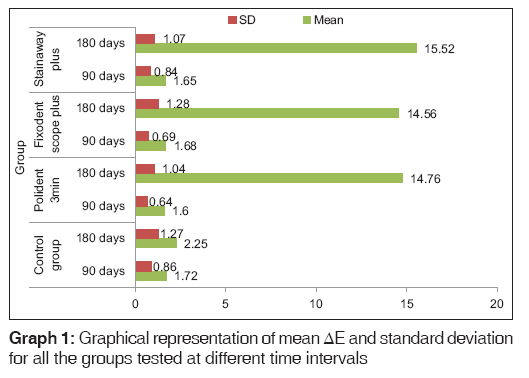
Table 1: Mean ΔE, standard deviation and National Bureau of Standards units for all the groups tested at different time intervals
The mean color change values (ΔE) are expressed in NBS units for all test groups at different time intervals in Table 1. The ΔE values of all heat-cure denture base resin samples increased with time. Critical marks of color change as suggested by the NBS units are tabulated in Table 2. Using NBS units to measure color change, all the groups demonstrated “slight change” or “noticeable change” for an immersion period of 90 days. However, all groups except for the control group exhibited “very much” color change following immersion for 180 days.
| Critical marks of color difference | Textile terms (NBS units) |
|---|---|
| Trace | 0.0-0.5 |
| Slight | 0.5-1.5 |
| Noticeable | 1.5-3.0 |
| Appreciable | 3.0-6.0 |
| Much | 6.0-12.0 |
| Very much | ≥12.0 |
NBS: National Bureau of Standards
Table 2: Critical marks of color difference as per the National Bureau of Standards
The paired t-test was performed to compare mean ΔE among time intervals (90 and 180 days) within all the groups tested [Table 3]. Highly significant values (P = 0.001) were observed for 180 days in all the test groups and the values observed within control group were statistically insignificant (P = 0.57).
| Groups | t | P | Inference |
|---|---|---|---|
| Group A | 1.35 | 0.57 | NS |
| Group B | 9.25 | 0.001 | HS |
| Group C | 11.81 | 0.001 | HS |
| Group D | 11.11 | 0.001 | HS |
HS: Highly significant, NS: Not significant
Table 3: Comparison of mean ΔE among time intervals (90 and 180 days) in all the groups using paired t-test
Comparison of mean ΔE among the groups at 90 and 180 days is done using ANOVA test [Table 4]. The color change observed among the groups within 90 days immersion interval were statistically insignificant (P = 0.91) and highly significant values (P = 0.001) were observed among the groups for 180 days immersion interval.
| Time period (days) | F | P | Inference |
|---|---|---|---|
| 90 | 0.372 | 0.91 | NS |
| 180 | 42.552 | 0.001 | HS |
HS: Highly significant, NS: Not significant
Table 4: Comparison of mean ΔE among the groups for 90 and 180 days using ANOVA test
The difference in the color change values for 180 days was evaluated using Dunnett’s T3 test [Table 5]. Highly significant values (P = 0.001) were noticed for all the test groups when compared with that of the control group.
| Time period (days) | Groups | P | Inference |
|---|---|---|---|
| 180 | Group A | ||
| Group B | 0.001 | HS | |
| Group C | 0.001 | HS | |
| Group D | 0.001 | HS | |
| Group B | |||
| Group C | 0.99 | NS | |
| Group D | 0.99 | NS | |
| Group C | |||
| Group D | 0.99 | NS | |
HS: Highly significant, NS: Not significant
Table 5: Comparison of mean ΔE between the groups for 180 days immersion interval using Dunnett’s T3 test
Of all the denture cleansers tested, although Group D demonstrated the greatest color change followed by Groups B and C for 180 days immersion interval, [Graph 1] these treatment comparisons were statistically insignificant (P = 0.99) as observed using Dunnett’s T3 test [Table 5].
Discussion
Oral candidiasis is most commonly seen associated with denture wearing individuals and is termed as denture stomatitis. Although denture stomatitis is multifactorial in nature, poor oral, and denture hygiene maintenance is the major predisposing factor. Thus, routine denture cleansing regimens with commercial denture cleansers are recommended to prevent the colonization of Candida albicans and its related species. The denture cleansing agents were also used to prevent plaque and extrinsic stain accumulation, etc.[7,8]
An ideal denture cleanser should be simple to use, effectively remove organic and inorganic matter from denture surface, have bactericidal and fungicidal properties, and be compatible with all denture base materials.[11] However, no current commercially available denture cleanser product fulfills all these requirements.
Denture cleansers include an effervescent component such as sodium perborate or sodium bicarbonate. When these effervescent tablets dissolve in water, sodium perborate decomposes to form an alkaline peroxide solution, which subsequently release oxygen and loosens debris by mechanical means. These effervescent compounds also reduce odor by neutralizing the by-products of bacteria.[12] However, Unlü et al. observed that the whitening of acrylic resin is the main disadvantage of denture cleansing agents.[7] Nikawa et al. also concluded that the high peroxide content and level of oxygenation in the strongly alkaline denture cleansing solution for prolonged duration is the main damaging factor for denture base materials.[13] Thus, these fast-acting cleansers were introduced to minimize the duration of soaking in denture cleansers.
To overcome the shortcomings of conventional overnight soaking, manufacturers altered the composition in denture cleansers by incorporating an additional ingredient, i.e., tetra acetyl ethylene diamine, (oxygen booster) which activates the oxidisers present in the cleanser to form peracetic acid resulting in strong antifungal and antibacterial action.[14] These newer cleansers thus can be used for shorter duration with maximum efficacy due to the presence of oxygen booster.
Complete dentures are fabricated using acrylic resin because of its low cost and relative ease of manipulation.[6] Ideally, denture base resins should have adequate physical and mechanical properties along with the ability to reproduce esthetics, matching natural color, and appearance of soft tissues. However, the long-term color stability of denture base materials is a significant problem in prosthodontics.[7] The color change of denture base resins is associated with various factors such as changes in the matrix of the material, staining effect of external colorants, i.e., food substances and colorants used in denture cleansers, etc., solubility, water sorption, leakage, surface roughness, and chemical degradation, etc.[8]
In the present study, minimal color change is seen in all the groups at 90 days immersion interval. Ferracane stated that the acrylic resins had a tendency to absorb solvent or water owing to the polarity of polymethylmethacrylate molecules. The solvent absorbed, then diffuses into the polymer network and disrupt the polymeric linkages causing hydrolytic degradation and formation of the acrylic zones with different optical properties. This change in optical properties results in the color change of acrylic resin.[15] This could probably be the reason for resin specimens demonstrating color change even in distilled water that is being used as a control in our study. However, the change in color of control and test group specimens is negligible and is not significant clinically. The results of this study are in accordance with the result of the studies conducted by Moon et al.[14] and Goiato et al.[16] wherein they observed the color change in acrylic specimens even after prolonged immersion in distilled water.
For 180 days immersion interval, all the test groups demonstrated greater color change compared to that of the control group. Although the color change was greater for Group D followed by Groups B and C, these changes were statistically insignificant.
Robinson et al. observed that the solvent present in denture cleansing solutions initially penetrates into the intermolecular polymer network and cause expansion of intermolecular spaces facilitating the leaching out of intrinsic pigments and the penetration of extrinsic colorants present in the denture cleansing solutions.[17] Thus, this could be the probable reason for the color change associated with all the test group specimens at 180 days interval compared to 90 days immersion interval.
Johnston and Kao observed that when the ΔE value is >3.7, it is no longer within the limits of clinical acceptability as the discoloration can be visualized by the naked eye. However, in a clinical scenario, as the color of entire denture base changes, it is difficult to detect a color change visually. Therefore, a clinically unacceptable level of color change would likely be higher than that corresponding to ΔE = 3.7.[18] In fact, most patients probably would not detect color changes corresponding to ΔE = 6.5 unless the changes occur over a long period of time along with mechanical and chemical degradation.[6] In this study, ΔE values of all the groups for 90 days time interval were ≤2.0, indicating that the color changes were minimal and clinically acceptable whereas the ΔE values for 180 days interval for all test groups except for control group was >12, indicating that these denture cleansers were clinically unacceptable. This probably could be due to the additional ingredient, tetra acetyl ethylene diamine, an activator that is being incorporated in these fast-acting cleansers.[14]
The results of the present study are in accordance with an in vitro study conducted by Hong et al.,[6] Peracini et al.[19] and Paranhos et al.[20] wherein they observed the color changes with increased immersion periods in overnight immersion type denture cleanser and the results observed in all the above studies were at clinically acceptable levels, i.e., ΔE ≤6.5 in contrast to the results observed in the present study using fast-acting cleansers.
Thus, the results of this study indicate that the color stability of the denture base acrylic resin was influenced by denture cleanser used and the immersion interval.
However, the present study has certain limitations as it could not completely simulate the oral environment since the results may vary due to the neutralizing effect of saliva, thermal changes of oral cavity and food, food colorants, microbes present in the oral cavity, etc., Furthermore, the dentures have biplanar surfaces which attract more stains rather than flat surface specimens altering the observed results. To overcome the limitations of this study denture actually used by patients could be evaluated.
Further investigations regarding the influence of denture cleanser on the physical and mechanical properties of denture base materials and additional studies on the correlation between the microbial activity of denture cleansers and their immersion intervals on acrylic resins are necessary to appreciate the effect of denture cleansers on the color stability of acrylic resins.
Conclusion
The results of this study demonstrate that long term usage of fast-acting denture cleansers could affect the color stability of denture base acrylic resins over a period of time. Thus, from our observations, we observed that the fast-acting denture cleansers are not suitable for daily denture care regimen for prolonged duration. However, these fast-acting cleansers were recommended in edentulous or partially edentulous persons with busy lifestyle and frequent travelers. Also the frequency, time period and temperature of water used for immersion could be altered to avoid detrimental effects on the denture base materials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- The glossary of prosthodontic terms. J Prosthet Dent 2005;94:10-92.
- Khindria SK, Mittal S, Sukhija U. Evolution of denture base materials. J Indian Prosthodont Soc 2009;9:64-9.
- Anusavice KJ. Phillips’ Science of Dental Materials. 11th ed. St.Louis WB Saunders Co., Elsevier; 2003.
- McCabe JF, Walls AW. Applied Dental Materials. 9th ed. Oxford Blackwell Publishing Ltd.; 2008.
- Sakaguchi RL, Powers RM. Craig’s Restorative Dental Materials. 13th ed. St. Louis: Mosby Inc., Elsevier; 2012.
- Hong G, Murata H, Li Y, Sadamori S, Hamada T. Influence of denture cleansers on the color stability of three types of denture base acrylic resin. J Prosthet Dent 2009;101:205-13.
- Unlü A, Altay OT, Sahmali S. The role of denture cleansers on the whitening of acrylic resins. Int J Prosthodont 1996;9:266-70.
- Moore TC, Smith DE, Kenny GE. Sanitization of dentures by several denture hygiene methods. J Prosthet Dent 1984;52:158-63.
- Wyszecki G, Stiles WS. Color Science: Concepts and Methods, Quantitative Data and Formulae. 2nd ed. New York: Wiley-Interscience; 1982. p. 13-116.
- Nimeroff I. Colorimetry. Natl Bur Stand Monogr 1968;104:4-32.
- Felton D, Cooper L, Duqum I, Minsley G, Guckes A, Haug S, et al. Evidence-based guidelines for the care and maintenanceof complete dentures. J Am Dent Assoc 1983;106:77-9.
- Felton D, Cooper L, Duqum I, Minsley G, Guckes A, Haug S, et al. Council on dental materials, instruments, and equipment.Denture cleansers. J Am Dent Assoc 2011;142 2 Suppl: 1s-20s.
- Nikawa H, Hamada T, Yamashiro H, Kumagai H. A review of in vitro and in vivo methods to evaluate the efficacy of denturecleansers. Int J Prosthodont 1999;12:153-9.
- Moon A, Powers JM, Kiat-Amnuay S. Color stability of denture teeth and acrylic base resin subjected daily to various consumer cleansers. J Esthet Restor Dent 2014;26:247-55.
- Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater 2006;22:211-22.
- Goiato MC, Nóbrega AS, dos Santos DM, Andreotti AM, Moreno A. Effect of different solutions on color stability of acrylic resin-based dentures. Braz Oral Res 2014;28. pii: S1806-83242013005000033.
- Robinson JG, McCabe JF, Storer R. The whitening of acrylic dentures: The role of denture cleansers. Br Dent J 1985;159:247-50.
- Johnston WM, Kao EC. Assessment of appearance match by visual observation and clinical colorimetry. J Dent Res 1989;68:819-22.
- Peracini A, Davi LR, de Queiroz Ribeiro N, de Souza RF, Lovato da Silva CH, de Freitas Oliveira Paranhos H. Effect of denture cleansers on physical properties of heat-polymerized acrylic resin. J Prosthodont Res 2010;54:78-83.
- Paranhos Hde F, Peracini A, Pisani MX, Oliveira Vde C, de Souza RF, Silva-Lovato CH. Color stability, surface roughness and flexural strength of an acrylic resin submitted to simulated overnight immersion in denture cleansers. Braz Dent J 2013;24:152-6.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.