Comparison of α-1 Antitrypsin Levels and other Inflammatory Markers in Smokers and Non-smokers
1Department of Biochemistry, King Abdulaziz University, Jeddah, Saudi Arabia
2Department of Biology, University of Jeddah, Jeddah, Saudi Arabia
3Ibn Sina National College for Medical Studies, College in Jeddah, Saudi Arabia
- *Corresponding Author:
- Dr. Nuha A. Alkhattabi
Department of Biochemistry
King Abdulaziz University
Jeddah, Saudi Arabia 21589
Tel: +966536665958
E-mail: naalkhattabi@kau.edu.sa
Citation:Alkhattabi N, et al. Comparison of α-1 Antitrypsin Levels and other Inflammatory Markers in Smokers and Non-smokers. Ann Med Health Sci Res. 2020;10: 865-869.
This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
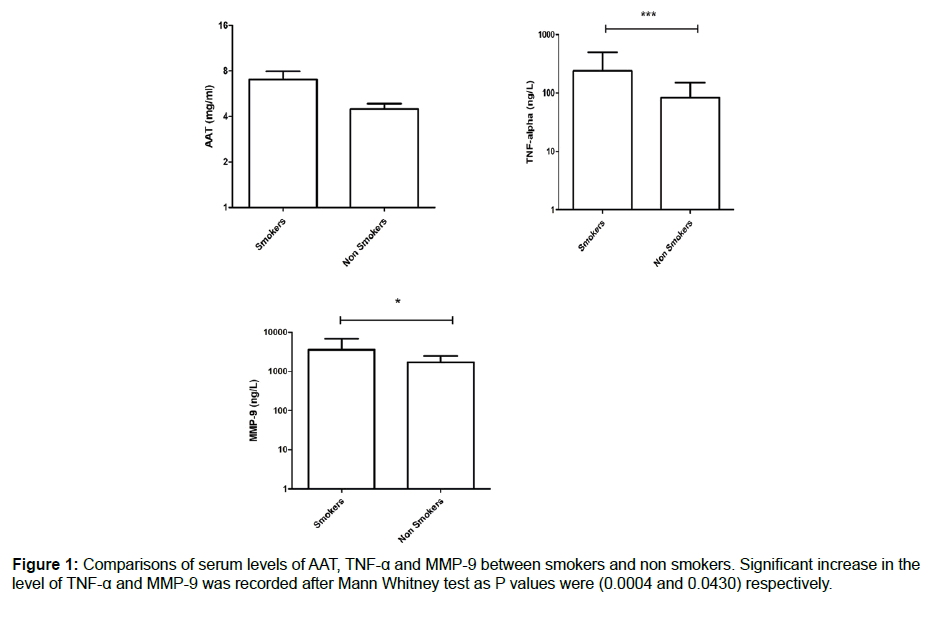
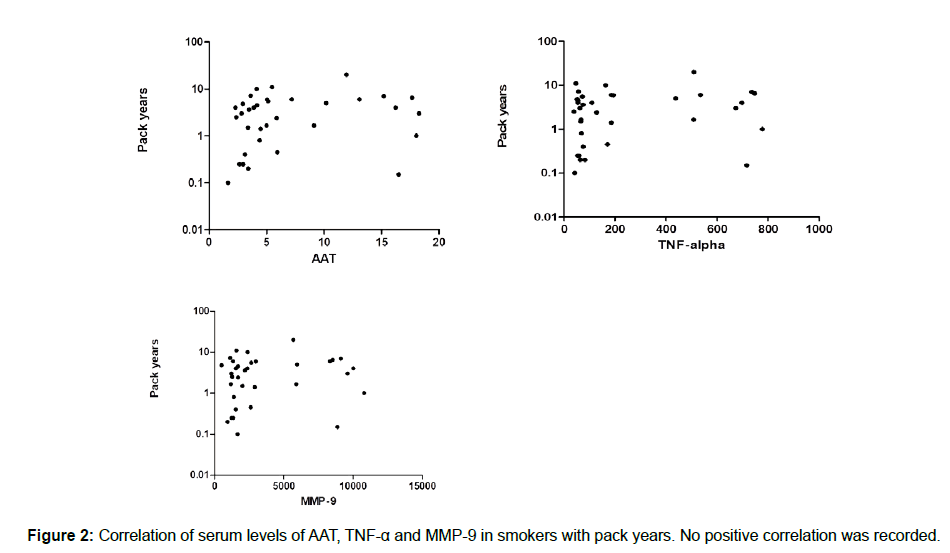
Background: With increased cigarette smoking worldwide, greater numbers of inflammatory diseases have been recorded. Coronary diseases, pulmonary diseases, and cancers of different types are all associated with cigarette smoking. In Saudi Arabia, people start smoking at an early age, which could lead to acceleration in immune system issues. The presence of biological parameters that could be used to diagnose possible inflammatory mechanisms would speed the discovery of new therapeutic approaches. Methodology: In this study, we examined the concentration of AAT, TNF-α and MMP9 in serums for smokers and nonsmokers in the young Saudi male population, aged 19 to 35 years old. Results: The recorded data showed a significant increase in both TNF-α and MMP-9, with mean ± SEM as 239.5 ± 43.14 vs. 83.02 ± 11.96 with P values of 0.0004 (3571 ± 542.5 vs. 1718 ± 137.3) and 0.0430, respectively. The AAT was higher in smokers compared with the control group, even though it did not reach a significant level (7.039 ± 093 vs. 4.465 ± 0.3901). We did not record any correlation between the measured parameters and packs/year of smokers. Conclusion: In conclusion, our results confirmed the possibility of using AAT, TNF-α and MMP-9 in the early diagnosis of immune system alterations associated with smoking. Understanding the mechanisms behind the development of chronic inflammatory disease would help in the prevention of complications and enable the discovery of therapeutic agents.
Keywords
Cigarette smoking; AAT; TNF-α; MMP-9; Inflammatory markers
Introduction
Cigarette smoking is a major cause of morbidity and mortality worldwide. [1] It is recognized as the second leading risk factor for death globally by the World Health Organization. [2] A study of 7,317 adults across 13 regions of Saudi Arabia found that 21.4% are smokers, and, of that sector, 32.9% are male and 3.9% are female. [3]
Additionally, exposure to tobacco smoke has been linked to the development of chronic diseases, including pulmonary diseases, heart infections, cancer, and transplant rejection. [4] Smoking is also a major risk factor for obstruction of airflow in chronic obstructive pulmonary disease (COPD) patients. [5] In addition, smoking influences the number of biological mediators of inflammation, leading to an immunosuppressant state. [6] In the lungs, smoking increases the number of white blood cells, including macrophages, neutrophils, mast cells, and eosinophil’s. It also alters macrophages’ and neutrophils’ function and decreases the number of airway dendritic cells. [7,8] Macrophages and neutrophils are the 2 major cells that release various proteolytic enzymes in excess of their natural inhibitors, leading to lung tissue destruction and trichomegaly. [9]
Some of the inflammatory mediators released by alveolar macrophages during an inflammatory state may play a role in the development of different diseases; these mediators include the matrix metalloproteinase (MMP) family, or MMP-9, most important for tissue remodeling; tumor necrosis factor-α (TNF-α); and α-1 antitrypsin (AAT).
AAT is known to be the most abundant circulatory serine protease inhibitor, secreted mainly by hepatocytes. Deficiency in AAT due to a genetic mutation can result in liver failure and lung disease. [10] In addition, AAT is also produced in lower concentrations by alveolar macrophages and delivered directly to the lungs, under the regulation of inflammatory mediators such as liposaccharides, IL-6, TNF-α, IL1-β and elastase in doses, depending on timing. [11,12]
In addition, Matrix metalloproteinase is a family of enzymes active in the remolding of extracellular matrix components. Gelatinase B (MMP-9) is controlled by various cytokines and cellular interactions; the activation of the pro-enzyme by a cascade of enzymes comprising serine proteases and other MMPs; and regulation by specific tissue inhibitors of MMPs (TIMPs) or by unspecific inhibitors, such as 2-macroglobulin. [13] Recently, reports have shown that there has been an increase in the expression of MMP-9 by alveolar macrophages in cigarette smokers, compared with nonsmokers. [14] Overexpression leads to degradation of the lung tissue, possibly a cause of COPD. [15]
In contrast, TNF-α is secreted by alveolar macrophages, which in turn may enhance the production of MMP-9. [16] TNF-α is one of the proinflammatory cytokines that plays a central role in the induction of pulmonary diseases. [17,18] Moreover, TNF-α regulates AAT, a neutrophil protease inhibitor.
Smoking may lead to the over production of neutrophil elastase, which causes tissue damage and lung emphysema. Importantly, AAT is central to the required balance of protease antiprotease, featured during the inflammatory response. Cigarette smoking may affect AAT production, which explains its role in the development of COPD. [19]
In this study, we aimed to measure the concentration of these 3 macrophage inflammatory markers— AAT, TNF-α and MMP- 9 in young male smokers to find a possible correlation among them as lung disease biomarkers.
Methods
Participants
In this study, we examined a total of 80 volunteers after getting their written consent. Overall, 36 nonsmokers and 44 smokers aged between 19 and 35 with a minimum of 2 years of smoking were included. All were reported healthy, with no known immunological, cardiovascular, or pulmonary disorders, according to the surveys collected.
Sample collection and analysis
We drew blood in a serum tube, clotted at room temperature for 30–40 min and then centrifuged at 2500 rpm for 15 min; the serum was immediately frozen at 2–4ºC for further analysis. All markers were analyzed using commercially available ELISA kits: TNF-α (Bioassay Technology Laboratory, Shanghai, China, (Cat. No. E0082Hu), MMP-9(Bioassay Technology Laboratory; Shanghai, China; (Cat. No. E0936Hu), and AAT (Bioassay Technology Laboratory; Shanghai, China; Cat. No. E0753Hu).
Statistical analysis
We analyzed the data using Graph Pad Prism software and presented all data as Mean ± SEM; we also utilized the Mann-Whitney U test for the comparison of means within each parameter in the 2 groups (smokers and control group). Finally, we performed a correlation test between the measured parameters and packs/year, considering data significant if the P value was ≤ 0.05.
Results
We measured the serum levels of AAT, TNF-α and MMP-9 and compared them in a group of smokers and a control group of nonsmokers. According to the data collected, there was a significant increase in the serum level of TNF-α in smokers compared with the control group (239.5 ± 43.14 vs. 83.02 ± 11.96), with a P value of 0.0004. In addition, the serum level of MMP-9 showed a significant increase in smokers compared to nonsmokers (3571 ± 542.5 vs. 1718 ± 137.3), with a P value of 0.0430. Finally, the serum AAT was higher in smokers compared with nonsmokers, but it did not reach a significant level (7.039 ± 0.893 vs. 4.465 ± 0.3901), as shown in Figure 1. As we looked at the measured inflammatory markers and the pack/year, we did not record any correlation, as shown in Figure 2.
Discussion
With the increase of cigarette smoking from an early age and an increase in studies indicating its role in the development of chronic diseases, it is important to highlight the possible mechanisms underlying its contribution to the progression of these health problems. Cigarette smoking is related to the development of immunological, cardiovascular, pulmonary, and different cancer types following prolonged consumption. [20] Young adults start smoking early, and the effect of cigarettes on their immunological response is a promising research area in which we could uncover possible alterations in their immunological systems and their related diseases. In this study, we aimed to analyze the alterations associated with some important inflammatory markers used as biomarkers for chronic inflammation.
AAT is a glycoprotein secreted by the liver in response to inflammation, functioning as a proteinase inhibitor. According to studies, the most important proteinase is neutrophil elastase, well-known for its role in the development of emphysema causing convective tissue destruction. AAT helps control the elastase activity, according to the proteinase/antiproteinase theory. [21] Many clinical diagnoseshave shown that individuals with deficiencies in this protein, a condition known as AATD, can develop emphysema and COPD. This disorder is genetically proven to be inherited. However, other factors such as cigarette smoking could be related to it. [22] In addition, AAT has been shown to have antiapoptotic potency against caspase 3, a cystine protease known as a regulator of programmed cell death. Lockett indicated the impairment of the antiapoptotic activity of AAT in smokers, which could explain the increased apoptosis of epithelial and alveolar macrophages in smokers with developed COPD. [23]
In this study, we compared the serum levels of AAT in younger smokers and nonsmokers to confirm the possibility of its use in the prediction of lung disease development. Sayyed showed the inhibitory effect of cigarette smoking on the serum levels of AAT in smokers, compared to levels in a control group, thus explaining its role in the development of COPD. [24] Our data suggested the possibility of increased AAT levels in smokers compared to nonsmokers. This supports Linja, who demonstrated an increase of AAT in the plasma of smokers, with or without COPD. [25] Additionally, we confirmed Serapinas’s results, showing increased serum levels of AAT in current smokers or former smokers, compared with a nonsmoking control group. These findings could also explain the role of AAT in the development of COPD; its increased levels in the blood resulting from systemic inflammation, meeting the elevated levels of alveolar neutrophil elastase, and proving to be a marker for increased risk of COPD development, as a result of low-grade lung inflammation. [26] Our participants were young smokers; elevated levels of AAT produced by the liver to protect the lungs of smokers against elastase would be an important diagnostic indicator of the risk of developing lung disease. In contrast, lower levels of AAT in individuals with AATD syndrome were also shown to be important for determining the development and severity of COPD, via either a shifting protease/antiprotease balance or an antiapoptotic effect on programmed cell death, as explained previously.
TNF-α is a well-known proinflammatory cytokine produced by a number of cells; the most important of these cells are macrophages. They are the first line of defense against pathogens and inflammatory mediators. The increased production of TNF by macrophages leads to the delivery of other cells in addition to neutrophils and macrophages to the lungs, thus aiding lung tissue destruction. [27]
Our results showed a significant increase in the serum TNF-α of smokers compared to the control group, corroborated by many studies. Petrescu showed elevated levels of TNF-α serum in smokers compared with a control group, demonstrating increasingly positive coloration with the number of packs/ day, which indicated an increased TNF-α level in smokers who consumed more than 1pack/day. [13] Moreover, other studies support the association of smoking with increased TNF-α production. [22] This may indicate the pro-inflammatory state development of smokers, which could be a biomarker for the development of pulmonary diseases.
MMP-9 is produced by activated T-cells, mast cells, macrophages, and vascular epithelial cells in response to such inflammatory mediators as cigarette smoke and reactive oxygen species. [28] A balance between metalloproteinase degradative enzymes and the TIMPs is required for normal lung functionality tissue remodeling and repair. Researchers have found that increased levels of these enzymes are associated with increased alveolar connective tissue degradation. [29] Our data showed a significant increase in systemic MMP-9 in smokers, compared with the control group, which could suggest it could be used as a biomarker for the development of lung abnormality and destruction. Atkinson also showed an increased level of MMP- 9 in smokers with emphysema correlated with the severity of emphysema; the researcher also pointed out, however, that it could not be used as a biomarker for the development of emphysema. [30] Other studies also demonstrated the correlation of cigarette smoking with elevated levels of MMP-9,as well as their association with emphysema development. [31,32]
Conclusion
In conclusion, all the biological parameters measured could be used as indicators for lung abnormality and pulmonary disease development. The presence of AAT, TNF-α, and MMP-9 has been proven to play an important role in normal lung tissue development; any associated alterations could result in pulmonary tissue damage and the subsequent development of COPD, emphysema, and other diseases that obstruct the airways. Assessment of alveolar health is required, but taking into account early signs of the presence of lung abnormality biomarkers and their development in young subjects alongside smoking habits could be useful for preventive and therapeutic discoveries.
Acknowledgment
The authors thank King Abdulaziz University and King Fahad Medical Research Center, Jeddah, Saudi Arabia
Competing Interests
The authors declare that they have no competing interests.
References
- West R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol Health. 2017;32:1018-1036.
- WHO. Global health risks: Mortality and burden of disease attributable to selected major risks. World Health Organization: Geneva. 2009.
- Algabbani AM, Almubark R, Althumiri N, Alqahtani A, BinDhim N. The Prevalence of Cigarette Smoking in Saudi Arabia in 2018. J Food and Drug Reg Sci. 2018;1:1.
- Warren GW, Sobus S, Gritz ER. The biological and clinical effects of smoking by patients with cancer and strategies to implement evidence-based tobacco cessation support. Lancet Oncol. 2014;15:e568-e580.
- Kang MJ, Oh YM, Lee JC, Kim DG, Park MJ, Lee MG, et al. Lung matrix metalloproteinase-9 correlates with cigarette smoking and obstruction of airflow. J Korean Med Sci. 2003;18:821.
- Gonçalves RB, Coletta RD, Silvério KG, Benevides L, Casati MZ, Da Silva JS, et al. Impact of smoking on inflammation: Overview of molecular mechanisms. Inflammation Research. 2011;60:409-424.
- Mehta H, Nazzal K, Sadikot RT. Cigarette smoking and innate immunity. Inflamm Res. 2008;57:497-503.
- Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2:372-377.
- Shapiro SD. Evolving concepts in the Pathogenesis of Chronic Obstructive Pulmonary Disease. Clin Chest Med. 2000;21:621-632.
- Hunt JM, Tuder R. Alpha 1 Anti-Trypsin: One Protein, Many Functions. Curr Mol Med. 2012;12:827-835.
- Bazzan E, Tinè M, Biondini D, Benetti R, Baraldo S, Turato G, et al. α1-Antitrypsin Polymerizes in Alveolar Macrophages of Smokers With and Without α-1-Antitrypsin Deficiency. Chest. 2018;154:607-616.
- Vant Wout EF, Van Schadewijk A, Savage ND, Stolk J, Hiemstra PS. Α-1-antitrypsin production by proinflammatory and anti-inflammatory macrophages and dendritic cells. Am J Resp Cell Mol. 2012;46:607-613.
- Opdenakker G, Van den Steen PE, Dubois B, Nelissen I, Van Coillie E, Masure S, et al. Gelatinase B functions as regulator and effector in leukocyte biology. J Leukoc Biol. 2001;69:851-859.
- Lim SA, Roche NI, Oliver BG, Mattos WA, Barnes PJ, Fan Chung K. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers: regulation by interleukin-10. Am J Respir Crit Care Med. 2000;162:1355-1360.
- Somborac-Bačura A, Popović-Grle S, Zovko V, Žanić-Grubišić T. Cigarette smoke induces activation of polymorphonuclear leukocytes. Lung. 2018;196:27-31.
- Petrescu F, Voican SC, Silosi I. Tumor necrosis factor-α serum levels in healthy smokers and nonsmokers. Int J Chronic Obstr. 2010;5:217.
- Thomas PS. Tumour necrosis factor‐α: The role of this multifunctional cytokine in asthma. Immunol Cell Biol. 2001;79:132-140.
- Dubravec DB, Spriggs DR, Mannick JA, Rodrick ML. Circulating human peripheral blood granulocytes synthesize and secrete tumor necrosis factor alpha. Proceedings of the National Academy of Sciences. 1990;87:6758-6761.
- Serapinas D, Narbekovas A, Juskevicius J, Sakalauskas R. Systemic inflammation in COPD in relation to smoking status. Multidiscip Resp Med. 2011;6:214.
- Onor IO, Stirling DL, Williams SR, Bediako D, Borghol A, Harris MB, et al. Clinical effects of cigarette smoking: epidemiologic impact and review of pharmacotherapy options. Int J Environ Res Public Health. 2017;14:1147.
- Stoller JK, Aboussouan LS. A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246-259.
- Mitra A, Vishweswaraiah S, Thimraj TA, Maheswarappa M, Krishnarao CS, Sundararaja Lokesh K, et al. Association of elevated serum GM-CSF, IFN-γ, IL-4, and TNF-α concentration with tobacco smoke induced chronic obstructive pulmonary disease in a South Indian population. Int J Inflammation. 2018;2018.
- Lockett AD, Van Demark M, Gu Y, Schweitzer KS, Sigua N, Kamocki K, et al. Effect of cigarette smoke exposure and structural modifications on the α-1 antitrypsin interaction with caspases. Mol med. 2012;18:445-454.
- Sayyed AK, Despande KH, Suryakar AN, Ankush RD, Katkam RV. Oxidative stress and serum α 1—Antitrypsin in smokers. Indian Journal of Clinical Biochemistry. 2008;23:375-377.
- Linja‐aho A, Mazur W, Toljamo T, Nieminen P, Ohlmeier S, Rönty M, et al. Distribution and levels of alpha‐1‐antitrypsin in the lung and plasma in smokers and chronic obstructive pulmonary disease. Apmis. 2013;121:11-21.
- Serapinas D, Narbekovas A, Juskevicius J, Sakalauskas R. Systemic inflammation in COPD in relation to smoking status. Multidiscip Resp Med. 2011;6:214.
- Demirjian L, Abboud RT, Li H, Duronio V. Acute effect of cigarette smoke on TNF-α release by macrophages mediated through the ERK1/2 pathway. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2006;1762:592-597.
- Sivaraman SK, Zachariah G, Annamala PT. Effect of smoking on metalloproteinases (MMPs) activity in patients with acute myocardial infarction (AMI). J Clin Diagn Res. 2014;8:27.
- Kang MJ, Oh YM, Lee JC, Kim DG, Park MJ, Lee MG, et al. Lung matrix metalloproteinase-9 correlates with cigarette smoking and obstruction of airflow. J Korean Med Sci. 2003;18:821.
- Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, et al. The role of matrix metalloproteinase-9 in cigarette smoke–induced emphysema. Am J Respir Crit Care Med. 2011;183:876-884.
- Louhelainen N, Stark H, Mazur W, Rytilä P, Djukanovic R, Kinnula VL. Elevation of sputum matrix metalloproteinase-9 persists up to 6 months after smoking cessation: a research study. BMC Pulmonary Medicine. 2010;10:13.
- Snitker S, Xie K, Ryan KA, Yu D, Shuldiner AR, Mitchell BD, et al. Correlation of circulating MMP-9 with white blood cell count in humans: Effect of smoking. PLoS One. 2013;8.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.