Comparison of Lipid Profile Levels in Tobacco Chewers with and without Leukoplakia – A Hospital Based Study
2 Department of Oral Medicine and Radiology, KLE VK Institute of Dental Sciences, KLE University, Belgaum, Karnataka, India
Citation: Radha A. Raut. Comparison of Lipid Profile Levels in Tobacco Chewers with and without Leukoplakia – A Hospital Based Study. Ann Med Health Sci Res. 2017; 7: 341-345
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: The most addictive constituent of tobacco is the alkaloid nicotine. Nicotine is said to have a considerable influence on the level of lipids in blood. Several studies have stated that alterations in lipid profile have been associated with development of OC and other premalignant lesions/conditions. Very little literature is available on lipid profile alterations at an early stage, i.e., before the development of OC. Aim: To assess and compare the lipid profile levels among tobacco chewers with and without leukoplakia. Materials & Methods: A total of 80 male subjects were included in the study and were randomly divided into two groups, Group I – 40 subjects (tobacco chewers without OL) and Group II – 40 subjects (tobacco chewers with OL). All the subjects who fulfilled the inclusion criteria were subjected to estimation of lipid profile. Results: The TC, HDL and LDL levels were higher, while TG and VLDL levels were lower in group II as compared to group I. Statistically significant difference was not noted between both the groups in relation to all the lipid parameters. The statistical analysis was carried out using Student’s unpaired t test and Chi – square test, with the help of Statistical Package for the Social Sciences (SPSS) 19 software. Conclusion: Thus, the lipid levels tend to increase in tobacco chewers and in subjects with OL, which can be an early change towards development of OC. This would help us in early diagnosis and prognosis of these lesions.
Keywords
Lipids; Tobacco; Oral Leukoplakia
Introduction
“Tobacco is a dirty weed. I like it. It satisfies no normal need. I like it. It makes you thin, it makes you lean, it takes the hair right off your bean. It’s the worst darn stuff I’ve ever seen. I like it.” - Graham Lee, Hemminger [1].
According to report of World Health Organization (WHO) tobacco is considered as the single greatest cause of preventable death globally [2].
The most addictive constituent of tobacco is the alkaloid nicotine, which is a stimulant and may develop tolerance and dependence in the users [3-5]. Nicotine present in tobacco is said to have a considerable influence on the level of lipids in blood [6-8].
Oral leukoplakia (OL) is the most common premalignant lesion, which plays an important role in pathogenesis of oral cancer (OC) [9]. Around 0.3% to 25% leukoplakias can undergo malignant transformation [10].
The etiology of OL is mostly related to the consumption of tobacco, alcohol, sanguinaria, ultraviolet radiation, certain viruses like human papilloma virus (HPV) 16 and HPV 18, trauma and other carcinogenic products. Among all these factors, tobacco is the major carcinogen which can lead to hyperkeratinization and can lead to an unrestrained proliferation and cell division. Patients using tobacco and with potentially premalignant lesions have been reported to show a significantly increased tendency to develop OC [11,12].
The lipids are a large and diverse group of naturally occurring organic compounds. The main biological functions of lipids include storing energy, signaling, and acting as major cell membrane components. They are essential for various biological functions including cell growth, division of normal and malignant tissues, activity of membrane-bound enzymes and stabilization of DNA helix and maintenance of the structural and functional integrity of all biological membranes [13-15]. The types of lipoproteins are – high density lipoprotein (HDL) which carries cholesterol out of the blood system, low density lipoprotein (LDL) which transports 75% of plasma cholesterol and very low density lipoprotein (VLDL) that enable fats and cholesterol to move within the water-based solution of the bloodstream [13].
The common oncogenic signaling pathways regulate the lipid metabolism in cancer cells and are believed to be important for the initiation and progression of tumors [16]. Several studies have stated that alterations in lipid profile have been associated with development of OC and other premalignant lesions/conditions [17-21]. Very little literature is available on lipid profile alterations at an early stage, i.e., before the development of OC.
A need was felt to understand the association of plasma total cholesterol (TC), TG, HDL, LDL and VLDL in tobacco chewers and in patients with OL. This would help us in early diagnosis and prognosis of these lesions. So, the present study was carried out to assess and compare the lipid profile levels among tobacco chewers with and without leukoplakia.
Methodology
Source of data
The subjects reporting to the Department of Oral Medicine and Radiology were selected for the study, based on the inclusion and exclusion criteria. The written informed consent was obtained from all the subjects. 80 Male subjects in the age group of 22 – 80 years were included in the study. The study was approved by the Ethical and Research Committee of the Institution.
All clinical examinations were carried out by a single trained examiner. Detailed case history including the height and weight to calculate Body Mass Index (BMI), diet history, habits history, extraoral and intraoral examination was performed as per the case history proforma.
The subjects with habit of tobacco chewing were selected based on their habit history and clinical examination. The subjects with provisional diagnosis of leukoplakia were subjected to incisional biopsy and were selected after histo-pathological confirmation of the lesion.
Method of selection of data
A total of 80 male subjects were included in the study and were randomly divided into two groups,
Group I – 40 subjects (tobacco chewers without OL) and
Group II – 40 subjects (tobacco chewers with OL).
Selection criteria
Inclusion criteria
• Subjects chewing tobacco for more than 5 times a day for atleast one year.
• Subjects with histopathologically diagnosed OL.
• Subjects willing to participate in the study.
Exclusion criteria
• Subjects suffering from any systemic illness and/or major illness in the past.
• Subjects with any deleterious habits other than tobacco chewing.
• Subjects with potentially malignant and/or premalignant conditions or lesions other than OL.
• Obese individuals with BMI more than 25.
• Non – vegetarians.
• Subjects consuming food with more than 2600 calories per day.
• Pregnant and lactating females.
All the subjects who fulfilled the inclusion criteria were subjected to estimation of lipid profile.
Complete lipid profile comprised of –
• Total cholesterol (TC)
• Triglycerides (TG)
• High density lipoprotein (HDL)
• Low density lipoprotein (LDL)
• Very low density lipoprotein (VLDL)
Procedure
• The lipid profile was estimated on same day using semi-automatic analyzer (Erba Chem 5 Plus, Mannheim®, Germany).
• Serum lipid values were estimated by mixing 0.01 ml serum sample with 1 ml of working reagent for individual lipids respectively.
• This mixture was incubated at 37°C for 10 minutes.
• For TC estimation (CHOD – PAP method) – The absorbance of standard and each test tube was read against respective reagent blank at 505/670 nm on bichromatic analyzer.
• For TG estimation (GPO – Trinder method) – The absorbance of standard and each test tube was read against respective reagent blank at 546/670 nm on bichromatic analyzer.
• For HDL estimation (Phosphotungstic acid method) – The absorbance of standard and each test tube was read against respective reagent blank at 546/670 nm on bichromatic analyzer.
• LDL and VLDL levels were calculated using Friedewald equation [22].
• Formula for LDL calculation –
LDL = Total cholesterol – (HDL + VLDL)
• Formula for VLDL calculation –
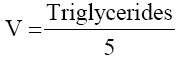
The data was presented as mean ± standard deviation (SD) for quantitative variables with normal distribution and significance of difference was accepted at p value < 0.05. The demographic data was summarized with descriptive statistics. The statistical analysis was carried out using Student’s unpaired t test for comparing lipid profile levels between the tobacco chewers with and without leukoplakia. Chi – square test was used to compare lipid profile levels in tobacco chewers with and without leukoplakia with the standard values. The statistical analysis was done using Statistical Package for the Social Sciences (SPSS) 19 software.
Results
The present study included 80 male subjects, who were divided into two groups, group I – 40 subjects, tobacco chewers without leukoplakia and group II – 40 subjects, tobacco chewers with leukoplakia.
No significant difference was identified between patients randomly assigned to the group I or group II with regard to age [Table 1].
| Group I | Group II | |
|---|---|---|
| Minimum | 22 | 27 |
| Maximum | 65 | 80 |
| Mean ± SD | 49.6 ± 9.30 | 53.8 ± 13.04 |
| p value | 0.094 | |
Table 1: Distribution of subjects according to age (in years).
Estimation of lipid profile levels
Statistically significant difference was not obtained on comparison of levels of TC, TG, HDL, LDL and VLDL in both the groups [Table 2].
| Lipid profile (mg/dl) |
Group I | Group II | p value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| TC | 214.3 | 62.75 | 223.1 | 51.12 | 0.494 |
| TG | 148.8 | 51.55 | 145 | 60.46 | 0.76 |
| HDL | 37.1 | 10.04 | 39.9 | 8.96 | 0.299 |
| LDL | 147.4 | 60.24 | 157.8 | 43.96 | 0.381 |
| VLDL | 29.6 | 10.09 | 28.9 | 12.09 | 0.764 |
Table 2: Comparison of two groups with respect to lipid profile levels by unpaired t test.
The percentage of subjects within the standard reference range with respect to serum TC, TG, HDL, LDL and VLDL levels were evaluated for both the groups based on the National Cholesterol Education Program (NCEP) guidelines for interpretation of lipid values, Adult Treatment Panel III (2001; updated 2004) as stated below -
Standard lipid profile (mg/dl):
Total cholesterol (TC)
• Desirable: < 200
• Borderline high: 200 - 239
• High: ≥ 240
Triglycerides (TG)
• Normal: < 150
• Borderline high: 150 - 199
• High: ≥ 200
HDL cholesterol (HDL)
• Low: < 40 (risk factor)
• Borderline high: 40 - 60
• High: > 60 (desirable)
LDL cholesterol (LDL)
• Optimal: < 100
• Borderline high: 100 - 159
• High: ≥ 160
VLDL cholesterol (VLDL)
• Optimal: ≤ 30
• High: > 30
The results obtained in the present study showed that the levels of TC were towards the higher levels in both the groups and on comparison, the TC, HDL and LDL levels were higher in group II as compared to group I. The levels of TG and VLDL were lower in group II as compared to group I. No statistically significant difference was noted between both the groups in relation to all the lipid parameters [Table 3].
| Lipids | Groups | Optimal | Borderline high | High | p value | |
|---|---|---|---|---|---|---|
| TC | I | 47.5 | 20 | 32.5 | 0.138 | |
| II | 32.5 | 40 | 27.5 | |||
| TG | I | 52.5 | 32.5 | 15 | 0.101 | |
| II | 67.5 | 12.5 | 20 | |||
| HDL | I | 67.5 | 30 | 2.5 | 0.809 | |
| II | 70 | 27.5 | 2.5 | |||
| LDL | I | 25 | 40 | 35 | 0.21 | |
| II | 1.8 | 47.5 | 42.5 | |||
| VLDL | I | 57.5 | - | 42.5 | 0.356 | |
| II | 67.5 | - | 32.5 | |||
Table 3: Distribution of subjects according to the standard range in both the groups with respect to serum TC, TG, HDL, LDL, VLDL levels.
The HDL levels tend to decrease, while LDL levels tend to increase in tobacco chewers and OL, but on comparison between both the groups, no statistically significant difference was noted [Table 3].
Discussion
Since ancient times, the phrase ‘Prevention is better than cure’ has always turned out to be very true. So to prevent development of any disease and for good prognosis of existing disease, its early diagnosis is very important.
The lipids are naturally occurring organic compounds that play an important role in cell growth, division of normal and malignant tissues, activity of membrane-bound enzymes and stabilization of DNA helix.13,14 Literature has stated that the lipid levels alter in oral precancerous lesions/conditions and oral cancer. The alterations in lipid profile have also been linked to tobacco use which is explained by a mechanism proposed by Brischetto et al. The release of adrenaline by the adrenal cortex is stimulated by nicotine, leading to the increased serum concentrations of free fatty acids. The free fatty acids stimulate hepatic synthesis and secretion of cholesterol, which leads to increase in lipid levels [5,23].
The free radicals and ROS generated by the tobacco carcinogens are responsible for high rate of oxidation/peroxidation of polyunsaturated fatty acids, leading to formation of end products like lipid hydroperoxides (LHP) and malondialdehyde (MDA). MDA levels indicate oxidative and cellular damage to tissues due to ROS and free radicals. MDA causes modulation of cell growth by activating signal transduction pathways, therefore acting as tumor promoters and co-carcinogenic agents. The levels of MDA increases in oral leukoplakia and oral squamous cell carcinoma patients reflecting interactions with various carcinogenic agents, which confirms increased lipid peroxidation and oxidative stress in these patients [24,25]. The oxidative stress can act in three possible ways. Firstly, ROS within the saliva could participate in the activation and inactivation of potentially carcinogenic compounds released from tobacco. Secondly, genotoxic damage can be caused in the oral mucosal cells by either ROS or ROS-activated carcinogens. Thirdly, ROS could conceivably react with salivary glycoproteins, causing degradation of the mucus and thus opening the underlying tissue to damage by genotoxic agents in the saliva. They also initiate mutagenic events by causing DNA damage that ultimately leads to degeneration of cellular components. In this way tobacco carcinogens can play an important role in development of oral precancerous lesions and OC [26,27].
Both biosynthetic and bioenergetic requirements change during malignant transformation of cells into cancer cells. Continuous de novo lipogenesis provides cancer cells with signaling lipid molecules, membrane building blocks and posttranslational modifications of proteins as well as energy supply to support rapid cell proliferation. Firstly, esterification of a number of endogenously synthesized fatty acids occurs to produce phospholipids, which facilitate the formation of detergent resistant membrane micro-domain for signal transduction, provide pivotal structural lipids, intracellular trafficking, polarization and migration required for cancer cells. Secondly, the newly generated lipid molecules mediate signal transduction in cancer cells. These lipids regulate a variety of cellular functions including cell proliferation, survival and migration by either activating other signaling proteins inside the cells, or by binding to a series of G protein-coupled receptors on the cell surfaces. Thirdly, the post-translational protein modification with lipid is also a vital process in regulating expression, localization and function of various signaling proteins [16].
The results obtained in the present study were in favor of study carried out by Goyal et al. [28]. who assessed serum lipid profile levels in subjects with tobacco habit and those with oral precancers. No statistically significant difference was obtained in values of serum lipid profile among both the groups. The explanation given was that the subjects in the tobacco habit group might not have used tobacco for long period of time and for required frequency and the patients in pre-cancer group might be at an initial stage, thus, unable to produce alterations in lipid levels [28].
HDL prevents lipid peroxidation on the cell membranes by counter balancing the oxidative damage caused by LDL. It has been suggested that HDL prevents both enzymatic and nonenzymatic generation of free radicals and thus acts as an anticarcinogen and a powerful antioxidant [29]. According to the results obtained in the present study, the HDL levels tend to decrease, while LDL levels tends to increase in tobacco chewers and OL. These findings suggest that tobacco use and presence of premalignant lesions like OL produces dyslipidemia. It can be an early indication towards progression to malignancy. Similar findings were obtained in studies carried out by Sharma SK et al. [3], Poorey et al. [30]., Reddy [31]., Meenakshisundaram et al. [32]., Patel et al. [17]., Khurana et al. [19]. etc.
The living cells acquire fatty acids for their metabolic demand by two sources, exogenous dietary source or by de novo endogenous synthesis. In most of the cancers, the lipid demand is fulfilled mostly by the endogenous source and the lipid metabolism is believed to be important for the initiation and progression of tumors and is regulated by the common oncogenic signaling pathways. A number of lipogenic enzymes utilize end products of glucose and glutamine metabolism, to synthesize fatty acids and their derivatives. Therefore, an exacerbated lipogenesis is noticed in cancer cells. The exacerbated lipogenesis is caused either by upregulation of lipid metabolizing enzymes or is directly coupled to other common metabolic pathways and their associated cell signaling pathways [16]. The results of present study showed higher levels of TC, TG, LDL, VLDL and lower levels of HDL in tobacco chewers and OL subjects suggesting that lipogenesis starts at an very early stage. This finding can be very useful for early diagnosis and further prognosis of the subjects.
A study was carried out by Kumar et al. 10]. to demonstrate lipid profile alterations in patients with OC and OL. They stated that there was an inverse relationship between serum lipid profile and OC, but they did not find any significant reduction in lipid profile among the pre-cancer group. Thus, hypolipidemia can be a late change occurring during carcinogenesis or can be an effect rather than the cause of cancer. So, the subject of major concern remains that whether hypolipidemia predisposes to cancer or is just an effect of cancer process. The present study should be carried out at molecular level, using a larger sample size to obtain more specific results.
Based on the results obtained in the present study, it can be suggested that hyperlipidemia precedes OC to meet the metabolic demands of cancer cells, while hypolipidemia can be a later effect of cancerous process.
Thus, it can be suggested that the lipid levels tend to increase in tobacco chewers and in subjects with OL, which can be an early change towards development of OC. The increase in lipid levels can be due to increase in metabolic demands of cells before the development of cancer. Leukoplakia can thus be considered responsible for changes in lipid levels in tobacco chewers. Further studies need to be carried out over a long period of time with more sample size and at molecular levels to assess the exact mechanism of dyslipidemia in tobacco users, oral precancerous lesions/conditions and OC.
Conclusions and Recommendation
In this study the overall incidence density rate (IDR) of death in the cohort was by far lower than other studies. However, incidence of death was still higher at the first few months of enrolment. The cumulative probability of survival and overall mean survival time was also comparable with other research. Treatment outcomes measured in terms of cure, death, and default rate were comparable to other reports as well. The main predictors of mortality among MDR-TB patients up on treatment were presence of comorbidities, adverse side effects, HIV sero-positivity and smaller baseline weight.
Therefore intervention to further reduce deaths has to focus on patients with comorbidities, severe side effects, HIV infected patients and lower base line body weight. The finding of this research may provide necessary information in areas of improvement; however further research is needed for giving policy level recommendations and addressing missed variables.
Acknowledgement
We acknowledge the guidance provided by all the staff of Department of Oral Medicine and Radiology for their valuable input.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Hemminger GH (1896-1949). 'Tobacco'. Penn State Froth. 1915 Nov.
- World Health Organization. WHO Report on the Global Tobacco Epidemic 2008? The MPOWER Package. Geneva: World Health Organization. 2008. ISBN 92-4-159628-7.
- Sharma SK, More UK. A comparative study of lipid profile in tobacco chewers in Pune district. Indian Journal of Public Health Research & Development. 2013;4:86-89.
- Singh S, Ramesh V, Premalatha B, Prashad KV, Ramadoss K. Alterations in serum lipid profile patterns in oral cancer. J Nat SciBiol Med. 2013;4: 374-378.
- Kamble PH, Rode MV, Phatak MS, Tayade P. Is smokeless tobacco using a risk factor for coronary artery disease? A comparative study of smokers and smokeless tobacco users. Int J App Basic Med Res. 2011;1:22-30.
- Rao Ch S, Subash YE. The effect of chronic tobacco smoking and chewing on the lipid profile. J ClinDiagn Res. 2013;7:31-34.
- Gepner AD, Piper ME, Johnson HM, Fiore MC, Baker TB, Stein JH. Effects of smoking and smoking cessation on lipids and lipoproteins: Outcomes from a randomized clinical trial. Am Heart J. 2011;161:145-151.
- Mammas IN, Bertsias GK, Linardakis M, Tzanakis NE, Labadarios DN, Kafatos AG. Cigarette smoking, alcohol consumption, and serum lipid profile among medical students in Greece. Eur J Public Health. 2003;13:278-82.
- Meisel P, Dau M, Sumnig W, Holtfreter B, Houshmand M, Nauck M, et al. Association between glycemia, serum lipoproteins, and the risk of oral leukoplakia: the population-based Study of Health in Pomerania (SHIP). Diabetes Care. 2010;33:1230-1232.
- Kumar P, Augustine J, Urs AB, Arora S, Gupta S, Mohanty VR. Serum lipid profile in oral cancer and leukoplakia: correlation with tobacco abuse and histological grading. J Cancer Res Ther. 2012;8:384-388.
- Greenberg MS, Glick M, Ship JA. Burket’soral medicine. (11th edn). Hamilton: BC Decker Inc; 2008. p.77-106.
- Lee JJ, Hong WK, Hittelman WN, Mao L, Lotan R, Shin DM, et al. Predicting cancer development in oral leukoplakia: ten years of translational research. Clin Cancer Res. 2000;6:1702-10.
- Mehrotra R, Pandya S, Chaudhary AK, Singh HP, Jaiswal RK, Singh M, et al. Lipid profile in oral submucous fibrosis. Lipids Health Dis. 2009 24;8:29.
- Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279:2610-2623.
- Bielecka-Dąbrowa A, Hannam S, Rysz J, Banach M. Malignancy-associated dyslipidemia. Open Cardiovasc Med J. 2011;5:35-40.
- Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem. 2012 26;3:167-174.
- Patel PS, Shah MH, Jha FP, Raval GN, Rawal RM, Patel MM, et al. Alteration in plasma lipid profile patterns in Head and Neck cancer and oral precancerous conditions. Indian J Cancer. 2004;41:25-31.
- Lohe VK, Degwekar SS, Bhowate RR, Kadu RP, Dangore SB. Evaluation of correlation of serum lipid profile in patients with oral cancer and precancer and its association with tobacco abuse. J Oral Pathol Med. 2010;39:141-148.
- Khurana M, Sharma D, Khandelwal PD. Lipid profile in smokers and tobacco chewers – a comparative study. J Assoc Physicians India. 2000; 48:895-897.
- Lopez-Jornet P, Camacho-Alonso F, Rodriguez-Martines MA. Alterations in serum lipid profile patterns in oral lichen planus: a cross-sectional study. Am J ClinDermatol. 2012;13:399-404.
- Husain B, Shreedhar B, Kamboj M, Natarajan S. A study on mast cell number and lipid profile in oral submucous fibrosis. Oral Health Dent Manag. 2014;13:412-417.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502.
- Brischetto CS, Connor WE, Connor SL, Matarazzo JD. Plasma lipid and lipoprotein profiles of cigarette smokers from randomly selected families: enhancement of hyperlipidemia and depression of high-density lipoprotein. Am J Cardiol. 1983;52:675-80.
- Metgud R, Bajaj S. Evaluation of salivary and serum lipid peroxidation, and glutathione in oral leukoplakia and oral squamous cell carcinoma. J Oral Sci. 2014;56:135-142.
- Li G, Da M, Zhang W, Wu H, Ye J, Chen J, et al. Alteration of serum lipid profile and its prognostic value in head and neck squamous cell carcinoma. J Oral Pathol Med. 2016;45:167-172.
- Stich HF, Anders F. The involvement of reactive oxygen species in oral cancers of betel quid/tobacco chewers. Mutat Res. 1989 Sep;214(1):47-61.
- Acharya S, Rai P, Hallikeri K, Anehosur V, Kale J. Serum lipid profile in oral squamous cell carcinoma: alterations and association with some clinico-pathological parameters and tobacco use. Int J Oral Maxillofac Surg. 2016;45:713-20.
- Goyal S, Ch L. Serum lipid profile in patients with oral tobacco habits and oral precancer lesions and conditions. WebmedCentralOral Medicine. 2013;4(2):WMC004034. Available from: doi: 10.9754/journal.WMC.2013.004034.
- Bielecka-Dąbrowa A, Hannam S, Rysz J, Banach M. Malignancy-associated dyslipidemia. Open Cardiovasc Med J. 2011;5:35-40.
- Poorey VK, Thakur P. Alteration of lipid profile in patients with head and neck malignancy. Indian J Otolaryngol Head Neck Surg. 2016;68:135-140.
- Reddy AV, Killampalli LK, Prakash AR, Naag S, Sreenath G, Biraggari SK. Analysis of lipid profile in cancer patients, smokers, and nonsmokers. Dent Res J (Isfahan). 2016;13:494-499.
- Meenakshisundaram R, Rajendiran C, Ponniah T. Lipid and lipoprotein profiles among middle aged male smokers: a study from southern India. TobInduc Dis. 2010;8:11.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.