Comparison of the Effects of Zonisamide, Ethosuximide and Pregabalin in the Chronic Constriction Injury Induced Neuropathic Pain in Rats
- *Corresponding Author:
- Dr. Sachin Goyal
Department of Pharmacology, Pacific College of Pharmacy, Pacific University, Udaipur - 313 024, Rajasthan, India.
E-mail: goyalsachin14@gmail.com
Abstract
Background: Evidence has been generated that various anticonvulsant agents provide relief of several chronic pain syndromes and therefore as an alternative to opioids, nonsteroidal anti‑inflammatory, and tricyclic antidepressant drugs in the treatment of neuropathic pain. The results of these studies thus raise the question of whether all anticonvulsant drugs or particular mechanistic classes may be efficacious in the treatment of neuropathic pain syndromes. Aim: The aim was to compare the clinically used anticonvulsant drugs which are differ in their mechanism of action in a chronic pain model, the chronic constriction injury, in order to determine if all anticonvulsants or only particular mechanistic classes of anticonvulsants are analgesic. Materials and Methods: The study included zonisamide, ethosuximide and pregabalin. All compounds were anticonvulsant with diverse mechanism of actions. The peripheral neuropathic pain was induced by chronic constriction injury of the sciatic nerve in male Sprague‑Dawley rats. Zonisamide (80 and 40 mg/kg), ethosuximide (300 and 100 mg/kg), pregabalin (50 and 20 mg/kg), and saline was administered intraperitoneally in respective groups in a blinded, randomized manner from postoperative day (POD) 7‑13. Paw withdrawal duration to spontaneous pain, chemical allodynia and mechanical hyperalgesia and paw withdrawal latency to mechanical allodynia and thermal hyperalgesia were tested before drug administration on POD7 and after administration on POD 7, 9, 11 and 13. Results: The present study suggests that these drugs could provide an effective alternative in the treatment of neuropathic pain. However, zonisamide and pregabalin appears to have suitable efficacy to treat a wide spectrum of neuropathic pain condition. Conclusion: The present findings suggest that the inhibition of N‑type calcium channels or voltage‑gated sodium and T‑type calcium channels provides better analgesic potential instead of inhibition of T‑type calcium channels alone.
Keywords
Chronic constriction injury model, Ethosuximide, Neuropathic pain, Pregabalin, Zonisamide
Introduction
Neuropathic pain is generally defined as a chronic pain state resulting from peripheral or central nerve injury either due to acute events (e.g., amputation, spinal cord injury) or systemic disease (e.g., diabetes, viral infection and cancer) and characterized by pathological symptoms, such as hyperalgesia and allodynia to mechanical and thermal (heat or cold) stimuli, as well as spontaneous pain.[1,2] Treatment of neuropathic pain, triggered by multiple insults to the nervous system, is a clinical challenge because the underlying mechanisms of neuropathic pain development remain poorly understood.[3,4]
Pharmacological treatments for chronic pain include opioids, nonsteroidal anti-inflammatory drugs and tricyclic antidepressants.[5,6] Neuropathic pain has been found to have a much-reduced sensitivity to the two major classes of analgesics, opioids and nonsteroidal anti-inflammatory drugs. Moreover, both opioids and nonsteroidal anti-inflammatory drugs produce problematic side-effects, particularly with prolonged use as may be required in the treatment of chronic pain, which markedly limit the clinical utility of these two classes of analgesics. Tricyclic antidepressants are more amenable to longer term use, but also produce side-effects, ranging from dry mouth to the potential for cardiotoxicity, which can limit their clinical utility.[7,8] Alternative pharmacological treatments for chronic pain are, therefore, needed.
Evidence has been generated over the past few decades that various anticonvulsant agents provide relief of several chronic pain syndromes, and therefore, as an alternative to opioids, nonsteroidal anti-inflammatory and tricyclic antidepressant drugs in the treatment of neuropathic pain.[9] Anticonvulsants such as Na+ channel blockers: Carbamazepine and phenytoin relieve neuropathic pain, but their usefulness is limited by low efficacy and adverse side-effects.[10] In particular, the N-type Ca2+ channel blocker pregabalin has been reported to be efficacious in diabetic peripheral neuropathy, postherpetic neuralgia and neuropathic pain associated with spinal cord injury.[11] Sodium and T-type Ca2+ channel blocker zonisamide is efficacious in the treatment of neuropathic pain.[12] Similarly, T-type Ca2+ channel blocker ethosuximide was found to be effective in chemotherapy evoked painful peripheral neuropathy.[13] It should be noted, however, that these reference compounds were mostly studied singularly (i.e., in separate studies), and that their efficacy was assessed under different experimental conditions and against different modalities using different behavioral readouts. The results of these studies thus raise the question of whether all anticonvulsant drugs or particularly classes may be efficacious in the treatment of neuropathic pain syndromes.
The major purpose of the present study was to compare clinically used anticonvulsant drugs which are differ in their mechanism of action in a chronic pain model, the chronic constriction injury, in order to determine if all anticonvulsants or only particular mechanistic classes of anticonvulsants are analgesic. The chronic constriction injury model appears to be one of the most frequently used model for the study of neuropathic pain and its treatment. The model, which is based on a unilateral loose ligation of the sciatic nerve, shows many of the pathophysiological properties of chronic neuropathic pain in human subjects.[14] It also shows a relatively high degree of similarity with other models of neuropathic pain in terms of the time course and the degree of allodynia and hyperalgesia against mechanical and/or thermal stimuli, the occurrence of spontaneous pain, and its sensitivity to sympathectomy.[15]
Materials and Methods
Animal and maintenance
Male Sprague-Dawley (SD) rats of body weight between 200 and 230 g were used for this work. All experiments were approved by the Institutional Animal Ethics Committee (1622/ PO/a/12/CPCSEA). Each rat was housed in plastic box cage individually with well-controlled supplied air, humidity (57%) and temperature under a 12 h light/dark cycle with food and water ad libitum.
Drugs and chemicals
Pregabalin was obtained from Torrent Research Centre (Gandhinagar, Gujarat), and zonisamide was obtained from Glenmark Pharmaceuticals (Mumbai, Maharashtra) as a gift sample. Ethosuximide was purchased from Sigma (India). All drugs were dissolved in isotonic saline solution to prepare drug solutions of specific concentration.
Induction of peripheral mononeuropathy: Chronic constriction injury model
Unilateral mononeuropathy was produced in rat using the chronic constriction injury (CCI) model performed essentially as described by Bennet and Xie.[14] The rats were anesthetized with intraperitoneal combination of ketamine and xylazine at 60 and 6.5 mg/kg, respectively. The left hind leg was shaved, moistened with a disinfectant and now common sciatic nerve was exposed at the level of the middle of the thigh by blunt dissection through the biceps femoris. Proximal to the sciatic trifurcation, about 7 mm of the nerve was freed of adhering tissues and four loose ligatures were made with 4.0 braided silk suture with about 1 mm spacing. The wound was then closed by suturing the muscle using chromic catgut with a continuous suture pattern. Finally, the skin was closed with 3.0 black braided silk sutures using a horizontal mattress suture pattern. Sham surgery was performed by exposing the sciatic nerve as described above, but not damaging it. Povidone-iodine ointment was applied topically to the wound and benzylpenicillin antibiotic (20,000 IU/Kg, b.i.d) was given intramuscularly for 5 days after surgery. The animals were then transferred to their home cage and left to recover.
Sensory testing (Nociceptive assay)
Nociceptive assays aimed at determining the severity of behavioral neuropathic parameters, namely spontaneous pain, allodynia, and hyperalgesia, were performed. The assays involved measurement of the degree of spontaneous (ongoing) pain and tests of hind limb withdrawal to cold, thermal and mechanical stimuli (dynamic mechanical allodynia, cold allodynia, mechanical and thermal hyperalgesia). Separate group of animals (n = 4) was used for each assay.
Spontaneous pain
Spontaneous pain was assessed for a total time period of 5 min as described previously by Choi et al.[16] The operated rats were individually placed inside an observation cage, and an initial acclimatization period of 10 min was given to each of the rat. A total of four rats were assigned to this group. Consider noted the cumulative duration that the rat holds its ipsilateral paw off the floor. The paw lifts associated with locomotion or body repositioning was not counted. For each measurement, three successive readings were taken without any elapse and the mean was calculated.
Dynamic component of mechanical allodynia
Dynamic allodynic response was assessed according to the procedure described by Field et al.[17] The operated rat was placed inside an observation cage. An initial acclimatization period of 10 min was given to each of the rat. A total of four rats were assigned to this group. A positive dynamic allodynia response consisted of lifting the affected paw for a finite period of time in response to mild stroking on the planter surface using a cotton bud. This stimulus is nonnoxious to a normally behaving rat. The latency to paw withdrawal was then noted. If no paw withdrawal was shown within 15 s, the test was terminated and animal was assigned nonresponsive. For each measurement, three successive readings were taken with 3 min elapsed between each test and mean was calculated.
Cold allodynia
The rats demonstrating unilateral mononeuropathy were assessed for acute cold allodynia sensitivity using the acetone drop application technique as described by Caudle et al.[18] The operated rat was placed inside an observation cage and allowed to acclimatize for 10 min. A total of four rats were assigned to this group. A few drops (100-200 ml) of freshly dispend acetone were squirted as a fine mist onto the midplanter region of the affected paw. A cold allodynic response was assessed by noting the duration of the paw withdrawal response. For each measurement, the paw was sampled 3 times with 3 min elapsed between each test and mean was calculated.
Mechanical hyperalgesia
Mononeuropathic rats were assessed for mechanical hyperalgesia sensitivity according to the procedure described by Gonzalez et al.[19] The operated rat was placed inside an observation cage and allowed to acclimatize for 10 min. A total of four rats were assigned to this group. Hind paw withdrawal duration (PWD) was measured after a mild pinprick stimulus to the midplanter surface of the ipsilateral hind paw. A withdrawal was defined as being abnormally prolonged if it lasted for at least 2 s. The mean duration of withdrawal was taken from a set of three responses with 3 min elapsed between each test.
Thermal hyperalgesia
Thermal hyperalgesia response was assessed according to the procedure described by Eddy and Leimbach.[20] A total of four rats were assigned to this group. The temperature of eddy’s hot plate was set at 55.0°C ± 0.1°C. The operated rats were placed on a heated surface and the time interval between placement and the shaking, licking or tucking of the affected hind paws was recorded as the latency response. If no paw withdrawal was shown within 22 s, the test was terminated and animal was assigned nonresponsive. For each measurement, three successive readings were taken with 3 min elapsed between each test and mean was calculated.
Experimental protocol
Eight groups, each comprising four SD rats, were employed in the present study. Baseline sensory response were measured for each group of animals (n = 4) according to predetermined manner. Animals showing all five neuropathic pain parameters in baseline measurement (0 h) on postoperative day 7 (POD7) were then administered the relevant drug by intraperitoneal route according to predetermined randomized table until POD13 and testing performed again after 1 h of drug administration. Each group of animals was used for only one drug administration and one parameter to ensure no “carry-over” effects. Pregabalin (50 and 20 mg/kg), zonisamide (80 and 40 mg/kg), and ethosuximide (300 and 100 mg/kg) were administered at t = 0 after baseline measurement. A vehicle control group was also run using saline. The treatment protocol remained the same for these seven groups. No drug testing was performed for sham-operated rats.
Group 1: CCI + vehicle.
Group 2: Sham control.
Group 3: CCI + Zonisamide 80 mg/kg.
Group 4: CCI + Zonisamide 40 mg/kg.
Group 5: CCI + Ethosuximide 300 mg/kg.
Group 6: CCI + Ethosuximide 100 mg/kg.
Group 7: CCI + Pregabalin 50 mg/kg.
Group 8: CCI + Pregabalin 20 mg/kg.
Statistical analysis
Data are presented as mean ± standard error of the mean Statistical significance was determined for drug effects by one-way Analysis of variance followed by Bonferroni post-hoc test for multiple comparisons. Comparison results with P < 0.05 were considered statistically significant. The statistical software package Statistical Package for the Social Sciences v. 17.0 (IBM SPSS, New York, USA) was used for the analysis.
Results
All animals included in this study exhibited characteristics neuropathic pain behavior in the baseline measurement (0 h) on POD7 after CCI surgery when compared with preoperative values except sham-operated animals.
Effect on spontaneous pain: Paw withdrawal duration
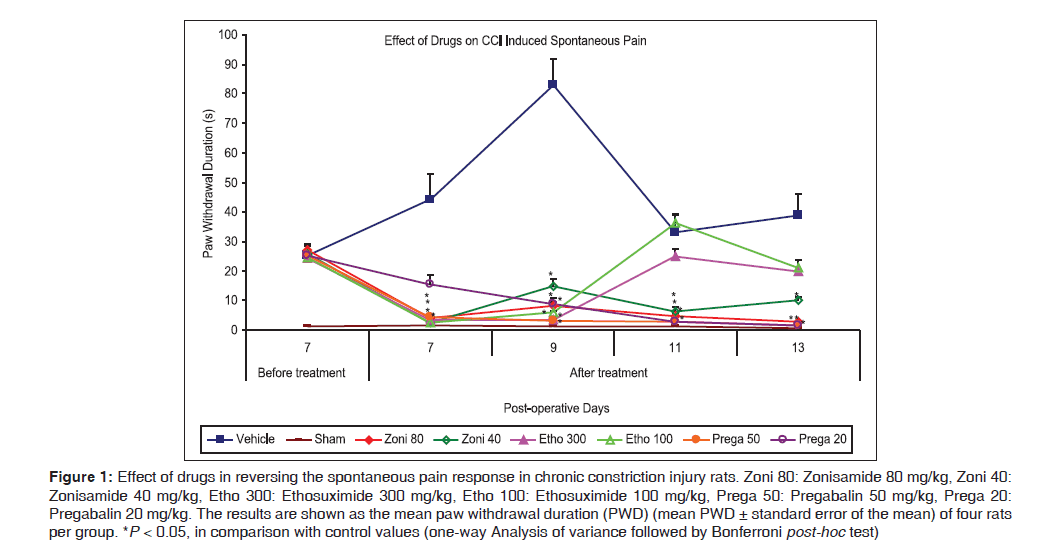
Administration of different drugs after baseline measurement on POD7 reversed the spontaneous pain response at respective doses when compared with control value, except by ethosuximide (300 mg/kg) and pregabalin (20 mg/kg) [Figure 1]. Zonisamide reversed the spontaneous pain response on POD7 at both dose levels (80 and 40 mg/kg, respectively) [4.0 (1.22) s and 2.5 (0.29) s, respectively, vs. 44.25 (8.74) s for control, P < 0.001] and the effect maintained during whole treatment period (POD7–13) on further dosing. Ethosuximide (300 mg/kg) was effective only on single time point, as at POD9 [3.5 (0.29) s vs. 83.0 (8.89) s for control, P < 0.001], while lower dose of ethosuximide (100 mg/kg) was observed to be effective on two time points, as at POD7 [2.5 (0.29) s vs. 44.25 (8.74) s for control, P < 0.001] and POD9 [6.0 (0.41) s vs. 83.0 (8.89) s for control, P < 0.001], during whole treatment period (PODs7–13). Parallely, pregabalin (50 mg/kg) was effectively attenuated spontaneous pain response after administration on POD7 [4.5 (0.65) s vs. 44.25 (8.74) s for control, P < 0.001], while pregabalin (20 mg/kg) showed effectiveness after three subsequent doses (from POD9, delayed effect) [8.75 (1.93) s vs. 83.0 (8.89) s for control, P < 0.001], and maintained the effect for remaining treatment period.
Figure 1: Effect of drugs in reversing the spontaneous pain response in chronic constriction injury rats. Zoni 80: Zonisamide 80 mg/kg, Zoni 40: Zonisamide 40 mg/kg, Etho 300: Ethosuximide 300 mg/kg, Etho 100: Ethosuximide 100 mg/kg, Prega 50: Pregabalin 50 mg/kg, Prega 20: Pregabalin 20 mg/kg. The results are shown as the mean paw withdrawal duration (PWD) (mean PWD ± standard error of the mean) of four rats per group. *P < 0.05, in comparison with control values (one-way Analysis of variance followed by Bonferroni post-hoc test)
Effect on mechanical allodynia: Paw withdrawal latency
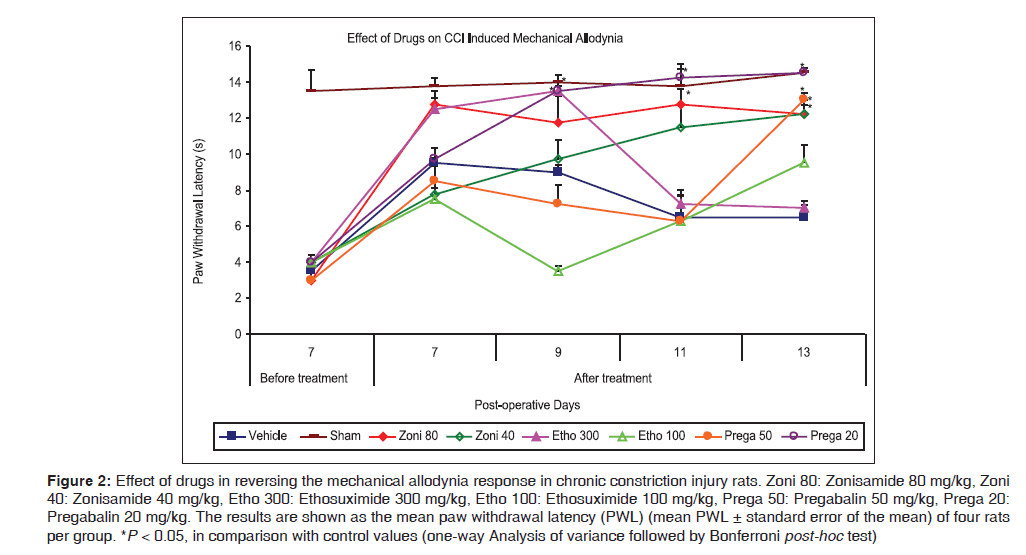
Figure 2 shows that administration of zonisamide (80 mg/kg) after baseline measurement on POD7 reversed the mechanical allodynia response only on two time point, as at POD11 [12.75 (0.85) s vs. 6.5 (1.55) s for control, P < 0.01] and POD13 [12.25 (0.48) s vs. 6.5 (0.65) s for control, P < 0.01], while lower dose of zonisamide (40 mg/kg) was effective only on single time point, as at POD13 [12.25 (0.75) s vs. 6.5 (0.65) s for control, P < 0.001]. Ethosuximide (300 mg/kg) was effective in reversing the allodynic response only on single time point, as at POD9 [13.5 (0.29) s vs. 9.0 (0.41) s for control, P = 0.04], while lower dose of ethosuximide (100 mg/kg) was ineffective on mechanical allodynia and did not reverse the allodynic response during entire treatment period. Present data indicated that pregabalin at dose level 50 mg/kg body weight was effectively attenuated mechanical allodynia response only on single time points,as at POD13 [13.0 (0.41) s vs. 6.5 (0.65) s for control, P < 0.001] during whole treatment period (PODs7–13). On other hand, the lower dose of pregabalin (20 mg/kg) showed more effectiveness in reversing the mechanical allodynia. It possessed antiallodynic effects from POD9 [13.5 (0.91) s vs. 9.0 (0.41) s for control, P = 0.04] and effect was maintained on continuous dosing on subsequent days till end of the treatment (POD13).
Figure 2: Effect of drugs in reversing the mechanical allodynia response in chronic constriction injury rats. Zoni 80: Zonisamide 80 mg/kg, Zoni 40: Zonisamide 40 mg/kg, Etho 300: Ethosuximide 300 mg/kg, Etho 100: Ethosuximide 100 mg/kg, Prega 50: Pregabalin 50 mg/kg, Prega 20: Pregabalin 20 mg/kg. The results are shown as the mean paw withdrawal latency (PWL) (mean PWL ± standard error of the mean) of four rats per group. *P < 0.05, in comparison with control values (one-way Analysis of variance followed by Bonferroni post-hoc test)
Effect on chemical allodynia: Paw withdrawal duration
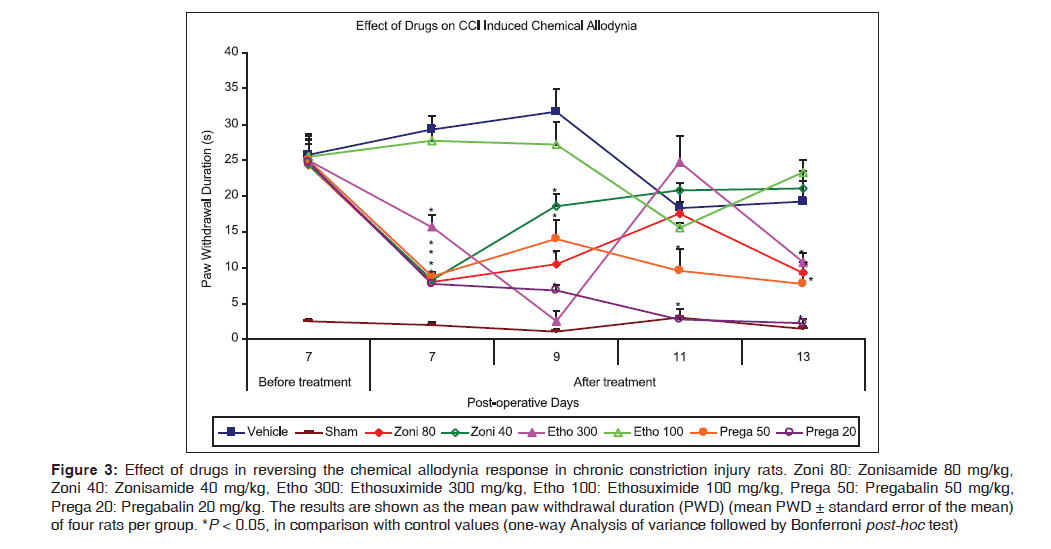
Figure 3 demonstrates the administration of zonisamide at dose level 80 mg/kg, after baseline measurement on POD7 reversed the chemical allodynia response [8.0 (1.29) s vs. 29.25 (1.93) s for control, P < 0.001] and maintained the effect on continuous dosing till POD13, except at POD11, while lower dose of zonisamide (40 mg/kg) showed effectiveness for limited period as at POD7 [8.25 (0.85) s vs. 29.25 (1.93) s for control, P < 0.001] and 9 [18.5 (1.71) s vs. 31.75 (3.12) s for control, P = 0.03]. Ethosuximide (300 mg/kg) was observed to be effective from POD7 [15.75 (1.65) s vs. 29.25 (1.93) s for control, P < 0.01] and effect was maintained on continuous dosing on subsequent days till end of the treatment (POD13) except on POD11, while ethosuximide 100 mg/kg, was found to be ineffective during whole treatment period (PODs7–13). Both doses of pregabalin (50 and 20 mg/kg, respectively) significantly attenuated chemically induced allodynia after administration on POD7 [8.75 (0.63) s and 7.75 (0.48) s, respectively, vs. 29.25 (1.93) s for control, P < 0.001] and observed to be effective during whole treatment period on further continuous dosing.
Figure 3: Effect of drugs in reversing the chemical allodynia response in chronic constriction injury rats. Zoni 80: Zonisamide 80 mg/kg, Zoni 40: Zonisamide 40 mg/kg, Etho 300: Ethosuximide 300 mg/kg, Etho 100: Ethosuximide 100 mg/kg, Prega 50: Pregabalin 50 mg/kg, Prega 20: Pregabalin 20 mg/kg. The results are shown as the mean paw withdrawal duration (PWD) (mean PWD ± standard error of the mean) of four rats per group. *P < 0.05, in comparison with control values (one-way Analysis of variance followed by Bonferroni post-hoc test)
Effect on mechanical hyperalgesia: Paw withdrawal duration
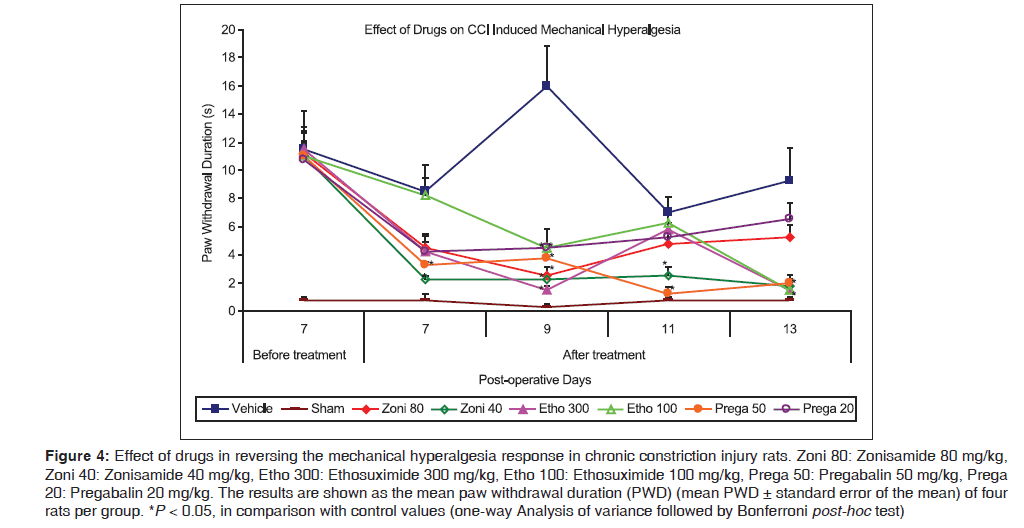
Figure 4 shows that hyperalgesia evoked by a mechanical pinprick stimulus was effectively attenuated by lower dose of zonisamide (40 mg/kg) on POD7 [2.25 (0.25) s vs. 8.5 (0.96) s for control, P < 0.01], and on further continuous dosing the effect was maintained till POD13, while higher dose (80 mg/kg) was observed to be effective only on single time point, as at POD9 [2.5 (0.65) s vs. 16.0 (2.86) s for control, P < 0.001]. Parallelly, the present data showed that both doses of ethosuximide (300 and 100 mg/kg, respectively) reversed mechanical hyperalgesia response only on single time point, as at POD9 [1.5 (0.29) s and 4.5 (0.29) s, respectively, vs. 16.0 (2.86) s for control, P < 0.001] during whole treatment period. Pregabalin (50 mg/kg) was effectively exerted antihyperalgesic activity after administration on POD7 [3.25 (0.25) s vs. 8.5 (0.96) s for control, P < 0.01] and maintained this pattern till POD13, while lower dose (20 mg/kg) was observed to be effective only on single time point, as at POD9 [4.5 (1.32) s vs. 16.0 (2.86) s for control, P < 0.001].
Figure 4: Effect of drugs in reversing the mechanical hyperalgesia response in chronic constriction injury rats. Zoni 80: Zonisamide 80 mg/kg, Zoni 40: Zonisamide 40 mg/kg, Etho 300: Ethosuximide 300 mg/kg, Etho 100: Ethosuximide 100 mg/kg, Prega 50: Pregabalin 50 mg/kg, Prega 20: Pregabalin 20 mg/kg. The results are shown as the mean paw withdrawal duration (PWD) (mean PWD ± standard error of the mean) of four rats per group. *P < 0.05, in comparison with control values (one-way Analysis of variance followed by Bonferroni post-hoc test)
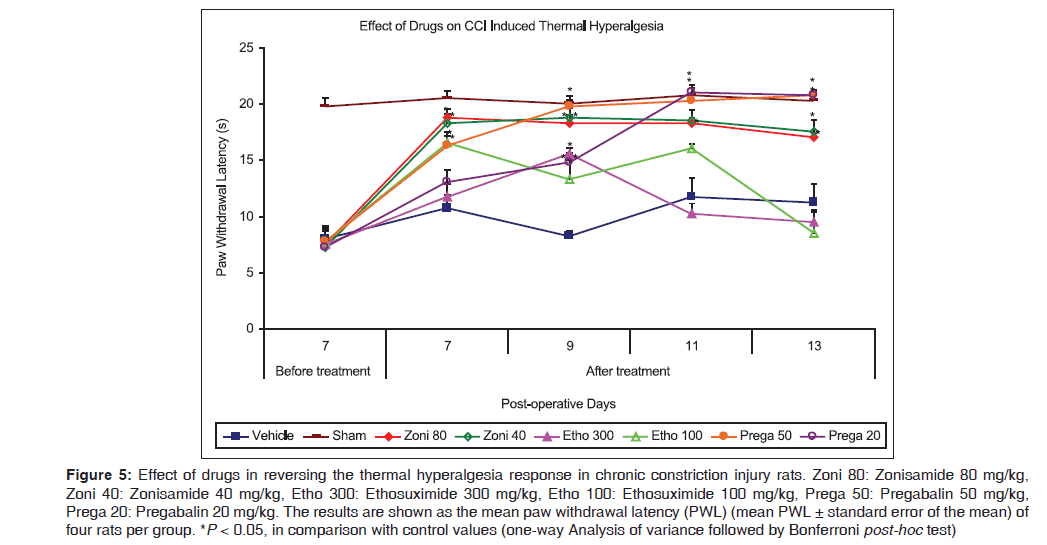
Effect on thermal hyperalgesia: Paw withdrawal latency
Figure 5 reflects hyperalgesia evoked by a thermal stimulus (hot plate) was effectively attenuated by both doses of zonisamide (80 and 40 mg/kg, respectively) after administration on POD7 [18.75 (0.75) s and 18.25 (0.85) s, respectively, vs. 10.75 (1.11) s for control, P < 0.01] and on further continuous dosing the effect was maintained during whole treatment period (PODs7–13). Parallely, both doses of ethosuximide (300 and 100 mg/kg) produced limited effect on thermal hyperalgesia during the treatment period (PODs7–13). Higher dose (300 mg/kg) was observed to be effective only single time point, as at POD9 [15.5 (0.65) s vs. 8.25 (0.48) s for control, P < 0.001], while lower dose (100 mg/kg) significantly reversed thermal hyperalgesia at two time points, as at POD7 [16.5 (0.65) s vs. 10.75 (1.11) s for control, P < 0.01] and POD9 [13.25 (1.75) s vs. 8.25 (0.48) s for control, P = 0.02]. Present results indicated that pregabalin (50 mg/kg) effectively attenuated hyperalgesia after administration on POD7 [16.25 (1.31) s vs. 10.75 (1.11) s for control, P = 0.02] and maintained this pattern during whole treatment period (PODs7–13). Similarly, pregabalin (20 mg/kg) showed antihyperalgesic activity after three subsequent doses (from POD9, delayed effect) [14.75 (0.85) s vs. 8.25 (0.48) s for control, P < 0.001] and maintained the effect for remaining treatment period.
Figure 5: Effect of drugs in reversing the thermal hyperalgesia response in chronic constriction injury rats. Zoni 80: Zonisamide 80 mg/kg, Zoni 40: Zonisamide 40 mg/kg, Etho 300: Ethosuximide 300 mg/kg, Etho 100: Ethosuximide 100 mg/kg, Prega 50: Pregabalin 50 mg/kg, Prega 20: Pregabalin 20 mg/kg. The results are shown as the mean paw withdrawal latency (PWL) (mean PWL ± standard error of the mean) of four rats per group. *P < 0.05, in comparison with control values (one-way Analysis of variance followed by Bonferroni post-hoc test)
Discussion
There has been growing interest in the potential utility of anticonvulsant drugs in the treatment of neuropathic pain, but systematic studies comparing the clinically used drugs have not been conducted. The present study, therefore, sought to directly compare the analgesic effects of clinically used anticonvulsant drugs with differing mechanisms of action by experimental peripheral neuropathy.
Inhibition of neuronal voltage-gated sodium and/or calcium channels may play a pivotal role in membrane excitability. Voltage-gated sodium channels (VGSCs) are critical elements of action potential initiation and propagation. Deregulation of VGSCs expression is thought to be involved in changes to neuronal firing and contribute to neuropathic pain states. Recently discovered T-type Ca2+ channels become activated after a small depolarization of the neuronal membrane and therefore play a crucial role in excitability of both central and peripheral neurons.[21-24] N-type Ca2+ channels are involved in neurotransmitter release and therefore play a dominant role in controlling nociceptive signal transmission under pathological conditions.[25] Neuronal injury produced by CCI, results in changes in sodium channel numbers and types, which contribute to the hyperexcitability,[23,26-28] while T-type calcium channels may regulate the rate of repetitive neuronal firing and N-type calcium channels may regulatethe release of neurotransmitters, further contributing to the hyperexcitability.[21,22,24,25,29] Blockade of sodium channels suppresses sodium-dependent action potential. Inhibition of, T-type calcium channels attenuates the sharp depolarization of the membrane potential while N-type calcium channels reduce the release of pronociceptive neurotransmitters from the central nerve terminals of primary afferent neurons.[23,25,30] The sodium and T-type Ca2+ channel blocker zonisamide has been reported to be efficacious in the treatment of neuropathic pain.[12] T-type Ca2+ channel blocker ethosuximide found to be effective in chemotherapy evoked painful peripheral neuropathy.[13] The N-type Ca2+ channel blocker pregabalin has been reported to be efficacious in diabetic peripheral neuropathy, postherpetic neuralgia, and neuropathic pain associated with spinal cord injury.[11]
In the present study, data were showed that CCI induced spontaneous pain was significantly attenuated by zonisamide and pregabalin in dose-dependent manner while ethosuximide showed limited effectiveness.
In case of mechanical allodynia, both doses of zonisamide (80 and 40 mg/kg), higher dose of pregabalin (50 mg/kg) and ethosuximide (300 mg/kg) showed limited effectiveness while lower dose of pregabalin (20 mg/kg) exerted delayed effect on CCI-induced mechanical allodynia. In the case of chemical allodynia, the higher dose of zonisamide and ethosuximide observed to be irregularly attenuated the chemically induced allodynic response while lower dose of zonisamide showed limited effectiveness. On the other hand, both doses of pregabalin significantly attenuated chemical allodynia. Lower dose of ethosuximide (100 mg/kg) did not reverse the CCI induced allodynic (mechanical and chemical) response.
In the case of hyperalgesia (mechanical and thermal), both doses of zonisamide were observed to be effective on thermal hyperalgesia while mechanical hyperalgesia was significantly attenuated by lower dose. Present data showed that the ethosuximide possessed limited antihyperalgesic activity at both doses. Pregabalin was exerted antihyperalgesic activity at higher dose while lower dose showed limited effect on mechanically induced hyperalgesia and delayed effect on thermal hyperalgesia.
Conclusion
The overall profile of anti-epileptic drugs used in this study suggests that these drugs could provide an effective alternative in the treatment of neuropathic pain. However, zonisamide and pregabalin appear to have suitable efficacy to treat a wide spectrum of neuropathic pain condition. The present findings suggest that the inhibition of N-type calcium channels or voltage-gated sodium and T-type calcium channels provide better analgesic potential instead of T-type calcium channels alone. Further research is required to confirm these findings in a large group of animals with special emphasis on molecular mechanisms of these compounds.
References
- Childers WE Jr, Baudy RB. N-methyl-D-aspartate antagonists and neuropathic pain: The search for relief. J Med Chem 2007;50:2557-62.
- McCarberg BH, Billington R. Consequences of neuropathic pain: Quality-of-life issues and associated costs. Am J Manag Care 2006;12:S263-8.
- Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: Risk factors and prevention. Lancet 2006;367:1618-25.
- Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci 2005;28:101-7.
- Pappagallo M, Haldey EJ. Pharmacological management of postherpetic neuralgia. CNS Drugs 2003;17:771-80.
- Collins SL, Moore RA, McQuayHJ, Wiffen P. Antidepressants and anticonvulsants for diabetic neuropathy and postherpetic neuralgia: A quantitative systematic review. J Pain Symptom Manage 2000;20:449-58.
- Arner S, Myerson B. Opioids in neuropathic pain. Pain Dig 1993;3:15-20.
- Max MB, Schafer SC, Culnane M, Dubner R, Gracely RH. Association of pain relief with drug side effects in postherpetic neuralgia: A single-dose study of clonidine, codeine, ibuprofen, and placebo. Clin Pharmacol Ther 1988;43:363-71.
- Backonja M. Anticonvulsants for the treatment of neuropathic pain syndromes. Curr Pain Headache Rep 2003;7:39-42.
- Hunter JC, Gogas KR, Hedley LR, Jacobson LO, Kassotakis L, Thompson J, et al. The effect of novel anti-epileptic drugs in rat experimental models of acute and chronic pain. Eur J Pharmacol 1997;324:153-60.
- Dworkin RH, Kirkpatrick P. Pregabalin. Nat Rev Drug Discov 2005;4:455-6.
- Krusz JC. Treatment of chronic pain with zonisamide. Pain Pract 2003;3:317-20.
- Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain 2004;109:150-61.
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988;33:87-107.
- Bridges D, Thompson SW, Rice AS. Mechanisms of neuropathic pain. Br J Anaesth 2001;87:12-26.
- Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain 1994;59:369-76.
- Field MJ, McCleary S, Hughes J, Singh L. Gabapentin and pregabalin, but not morphine and amitriptyline, block both static and dynamic components of mechanical allodynia induced by streptozocin in the rat. Pain 1999;80:391-8.
- Caudle RM, Mannes AJ, Benoliel R, Eliav E, Iadarola MJ. Intrathecally administered cholera toxin blocks allodynia and hyperalgesia in persistent pain models. J Pain 2001;2:118-27.
- Gonzalez MI, Field MJ, Hughes J, Singh L. Evaluation of selective NK (1) receptor antagonist CI-1021 in animal models of inflammatory and neuropathic pain. J Pharmacol Exp Ther 2000;294:444-50.
- Eddy NB, Leimbach D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J Pharmacol Exp Ther 1953;107:385-93.
- Dogrul A, Gardell LR, Ossipov MH, Tulunay FC, Lai J, Porreca F. Reversal of experimental neuropathic pain by T-type calcium channel blockers. Pain 2003;105:159-68.
- Perez-Reyes E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 2003;83:117-61.
- Waxman SG, Dib-Hajj S, Cummins TR, Black JA. Sodium channel and their genes: Dynamic depression in the normal nervous system, disragulation in the disease states. Brain Res 2000;886:5-14.
- Huguenard JR. Low-threshold calcium currents in central nervous system neurons. Annu Rev Physiol 1996;58:329-48.
- McGivern JG. Targeting N-type and T-type calcium channels for the treatment of pain. Drug Discov Today 2006;11:245-53.
- Baron R. Peripheral neuropathic pain: From mechanisms to symptoms. Clin J Pain 2000;16:S12-20.
- Cummins TR, Dib-Hajj SD, Black JA, Waxman SG. Sodium channels and the molecular pathophysiology of pain. Prog Brain Res 2000;129:3-19.
- Dib-Hajj SD, Fjell J, Cummins TR, Zheng Z, Fried K, LaMotte R, et al. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain 1999;83:591-600.
- Perret D, Luo ZD. Targeting voltage-gated calcium channels for neuropathic pain management. Neurotherapeutics 2009;6:679-92.
- Jagodic MM, Pathirathna S, Joksovic PM, Lee W, Nelson MT, Naik AK, et al. Upregulation of the T-type calcium current in small rat sensory neurons after chronic constrictive injury of the sciatic nerve. J Neurophysiol 2008;99:3151-6.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.