Correlation of Biochemical Variables to Obesity and Prediction of Cardiovascular Risk Factors in Children
Citation: Bindu T Nair. A Study of the Correlation of Personality Traits (Neuroticism and Psychoticism) and Self-efficacy in Weight Control with Unhealthy Eating Behaviors and Attitudes. Ann Med Health Sci Res. 2017; 7: 39-43
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: Incidence of obesity in early childhood is increasing nowadays because of sedentary lifestyle, faulty food habits and lack of exercise. It has been observed that a variety of cardiovascular risk factors are closely linked to various biochemical variables in overweight and obese children. Objectives: Our study endeavoured to observe the correlation of various biochemical variables to cardiovascular risk factors in obese children. Materials and Methods: Ours was a case control study conducted on school children aged five to fifteen years presenting to Out Patient Department (OPD) of a 999 bedded large hospital in North India. These children were visiting the hospital for non-specific illnesses or for routine health checkup. Fifty consecutive obese and non-obese children were taken up for the study. Subjects with any chronic illnesses or on any chronic medications were excluded. Results: There was no significant difference in obesity with regard to age and gender between the study group and the control group (p=0.846 and p=0.567, respectively). There was significant statistical difference between the obese and control group children for both systolic as well as diastolic BP. In our study, Body mass index (BMI) was inversely associated with HDL cholesterol. Conclusion: Increased blood pressure, lipid and glucose abnormalities are associated with an increased cardiovascular risk in children. Hence, preventive measures to control obesity should be taken from childhood to save our future adults and prevent economic drain of the country.
Keywords
Obesity; Lipid profile; Blood sugar; Systolic and diastolic blood pressure; Cardiovascular risk; Children
Introduction
World Health Organization (WHO) has confirmed that obesity has assumed proportions of an epidemic all over the world including developing countries. [1]. It is considered a chronic childhood disease which is more dangerous than even malnutrition as it is associated with a large number of comorbidities and mortality. Over the past 3 decades, the prevalence of childhood obesity has increased remarkably all over the world. The growing prevalence of childhood obesity has also led to appearance of obesity-related comorbid disease entities at an early age.
The problems associated with obesity in children include social issues like prejudice and psychosocial problems including low self-esteem which in turn affects the academic performance of the child and also relationship with friends and peer group. Childhood obesity if not checked would lead to metabolic syndrome of adulthood have a spectrum of central obesity, dyslipidaemia, hypertension and insulin resistance. [2,3]. These form a cluster of strong cardiovascular risk factors leading to morbidity and mortality of young adults.
It is a major contributor to increasing healthcare expenditures in developed as well as developing countries. Therefore, it is important to prevent childhood obesity and to identify overweight and obese children at an early stage so that adequate measures are taken to facilitate these children attain and maintain a healthy weight.
Objective
To investigate the effect of body mass index on various biochemical variables and their correlation to various cardiovascular disease risk parameters in overweight and obese children.
Materials and Methods
Study population and design
Ours was a case control study conducted on school children aged five to fifteen years presenting to OPD of a 999 bedded large hospital in North India. Subjects with any chronic illnesses or on any chronic medications were excluded.
The study group consisted of 50 consecutive obese children and the control group included 50 age and gender-matched healthy children. These children were visiting the hospital for non-specific illnesses or for routine health check-up. Fifty consecutive obese and non-obese children were taken up for the study. Depending upon the BMI, these subjects were divided into two groups: Obese group with BMI of ≥ 30 kg/m2 and nonobese group with BMI of 18.5-22.9 kg/m2. [4,5].
Exclusion criteria
Children excluded in both groups were those with history of any chronic illnesses like any cardiopulmonary or renal diseases, or those on any medications for a long duration. Children with history of any major surgery (cardiac, pulmonary or abdominal) related to obesity or subjects undergoing any physical conditioning program were also excluded. A detailed medical history was obtained and a thorough physical examination (including evaluation for syndromes and endocrine diseases) was performed so that any unidentified concomitant disease is excluded.
Complete blood count and biochemistry including thyroid function tests and serum cortisol were done to rule out any underlying unidentified pathology. Children with obesityrelated syndromes such as Prader-Willi syndrome, Laurence- Moon-Biedl syndrome, etc. and endocrine disorder such as Cushing’s syndrome, hypothyroidism, etc. were excluded from the study.
Data collection was carried out from participants who matched inclusion criteria and had written parental consent. Two post graduate paediatric residents who had undergone training on anthropometric measurements examined the subjects. Weight and height measurements were done with the children wearing light clothes and no footwear. Weight was recorded to the nearest 0.5 kg with clothing using a standard weighing machine. Height was measured to the nearest 1 cm without footwear. The BMI or Quetelet Index was conventionally calculated as weight in kg/height (in meters2) for each subject. [6,7].
The study protocol was reviewed and approved by the hospital ethics committee. The venous blood samples were collected in the departmental laboratory between 8 to 10 am. Evaluation of primary cardiovascular risk factors – systolic blood pressure (SBP), diastolic blood pressure (DBP), high density lipoproteins (HDL) and fasting glucose was done.
Arterial blood pressure was measured manually using a mercury sphygmomanometer with a suitable cuff size for each participant after a 5-minute rest in the supine position. SBP was determined by the onset of the tapping Korotkoff sound while DBP was determined after the disappearance of the Korotkoff sound. The average of six measurements (three taken by each of two examiners) with a mercury sphygmomanometer was used in all analyses. The fourth Korotkoff phase was considered the diastolic blood pressure. [8].
For lipid profile and liver function test, 3 ml of blood was collected from each subject after overnight fasting of 12 hours. Serum values of total cholesterol (TC), high density lipoprotein (HDL), low density lipoproteins (LDL), and very low density lipoprotein (VLDL), triglycerides (TG) were measured by enzymatic method using Semi Auto analyzer (Mispa Excel Chemistry Analyser). Concentration was represented in mg/ dl. Estimation of fasting blood sugar (FBS) was done by using medical device (One Touch Glucometer).
Statistical analysis
All data was entered and analyzed using Statistical Package for Social Sciences version 15.0 (SPSS, Inc., Chicago, IL, USA). For continuous data, descriptive analyses used was mean and standard deviation (SD). Comparisons between the two study groups were carried out with two-tailed Student’s t-test for normally distributed continuous variables, Mann-Whitney U test for continuous variables without normal distribution, and chi-square test for dichotomous variables. P values below 0.05 were considered as significant.
Results
Out of the 100 children enrolled in the study, 54 were boys and 46 were girls. Out of these 54 boys, 28 boys were obese and 26 were non-obese. Amongst the 46 girls, 22 were obese while remaining 24 were non-obese. Amongst the 50 consecutive obese children (28 males and 22 females), the mean age was 10.7 ± 2.4 years and the control group
(Which included 50 age and gender-matched healthy children of 26 males and 24 females) had a mean age of 11 ± 2.96 years.
There was no significant difference in obesity with regard to age and gender in both the control and study groups (p=0.846 and p=0.567, respectively). Demographic, anthropometric and biochemical characteristics of the participants are given in Table 1.
| Parameters | Obese Children | Control Group | p-value |
|---|---|---|---|
| Age (Years) | 10.7 ± 2.4 (8-15) | 11 ± 2.96 (8-15) | 0.846 |
| Gender | |||
| Male | 28 | 26 | 0.567† |
| Female | 22 | 24 | |
| Height (Cm) | 167.33 ± 9.22 | 164.65 ± 9.32 | 0.267 |
| Weight (Kg) | 58.70 ± 7.93 | 73.93 ± 11.10 | <0.01 |
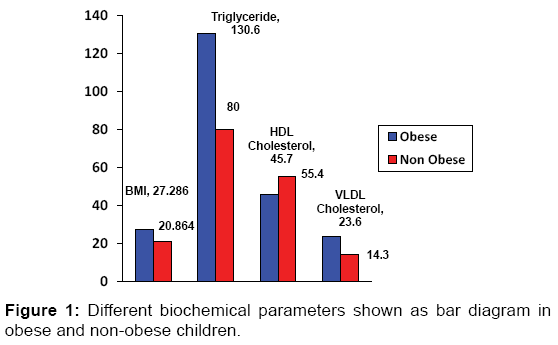
| BMI | 31.286 ± 2.8473 | 20.864 ± 1.8113 | <0.001* |
| SBP mmHg | 109.4 ±6.8 | 100.9 ± 11.9 | <0.001* |
| DBP mmHg | 67.1 ± 4.6 | 62.4 ± 5.9 | <0.001* |
| Serum Fasting Glucose(mg/dl) | 90.5 ± 9.4 (70-104) | 88.1 ± 8.8 (70-103) | 0.122* |
| Total cholesterol (mg/dL) | 175.8 ± 37.8 | 165.12 ± 24.1 | 0.176* |
| Triglyceride (mg/dL) | 130.6 ± 69.7 | 80 ± 39.8 | <0.001‡ |
| HDL-cholesterol (mg/dL) | 45.7 ± 13.1(34-68) | 55.4 ± 13 (42-71) | 0.005* |
| LDL-cholesterol (mg/dL) | 101.4 ± 28.5 (60-158) | 92.2 ± 26.8 (56-142) | 0.192* |
| VLDL-cholesterol (mg/dL) | 23.6 ± 11.2 (11-52) | 14.3 ± 6.5 (8-30) | <0.001‡ |
| AST (IU) | 23.6 ± 6.2 (11-42) | 20.5 ± 7.5 (13-32) | 0.064* |
| ALT (IU) | 27.4 ± 10.2(12-68) | 25.2 ± 11.2(11-46) | 0.092* |
| GGT (IU) | 29.3 ± 14.7 (15-49) | 24.8 ± 13.7 (13-42) | 0.213‡ |
| BMI SDS: Body Mass Index Standard Deviation Score; HDL: High-Density Lipoprotein; LDL: Low-Density Lipoprotein; VLDL: Very Low-Density Lipoprotein; AST: Aspartate Aminotransferase; ALT: Alanin Aminotransferase; GGT: Gamma GlutamylTransferase. *: Two-Tailed Student’s T-Test; †: Chi-Square Test; ‡: Mann-Whitney U Test. Values Represent Mean ± Standard Deviation and Minimum and Maximum In Parentheses | |||
Table 1: Demographic, clinical and biochemical values in children of obese and control groups.
There was significant statistical difference between the obese and control group children for both systolic as well as diastolic BP. Obese children had significantly higher anthropometric parameters and worse biochemical profiles (BMI, TG and VLDL, p<0.001] compared to normal-weight children [Figure 1]. HDL was found to be lower in obese children as compared to non-obese children which was also statistically significant (p<0.005). Other biochemical variables with cardiovascular risks like total cholesterol and LDL when compared in both the study groups were not significant.
We compared evidence of non-alcoholic fatty liver disease (NAFLD) risks in both the study groups of children as NAFLD is quite commonly seen in obese adults. Liver function tests like aspartate aminotransferase (AST), alanine aminotransferase (ALT) and gamma glutamyl transferase (GGT) was tested in both the study groups. However, though the AST and ALT was higher in obese children compared to non–obese children but they were not significant as seen in studies comparing obese adults versus non-obese adults. [9-11].
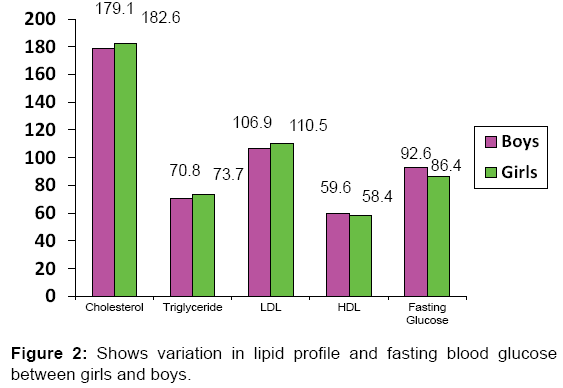
BMI was positively associated with systolic blood pressure, diastolic blood pressure and triglycerides in other studies also. [12-14]. As in our study, BMI was inversely associated with HDL cholesterol as seen in other studies also. [15-17]. BMI was also associated with cardiovascular disease risks and associated biochemical parameters. [18-20]. Various studies have also found metabolic syndrome to be present in statistically significant number of obese patients. [21]. There was no significant difference between obese girls and boys in any biochemical parameters like Total cholesterol, TG, LDL, HDL [Figure 2] except for fasting blood sugar which was higher in obese male children [Table 2].
| Parameter | Boys (n=28; 56%) | Girls (n=22: 44%) | p-value |
|---|---|---|---|
| Fasting Glucose (mg/dL) | 92.6 (9.6) | 86.4 (11.4) | 0.001 |
| Cholesterol (mg/dL) | 179.1 (24.3) | 182.6 (27.4) | NS |
| Triglyceride (mg/dL) | 70.8 (23.1) | 73.7 (24.5) | 0.029 |
| HDL-Cholesterol (mg/dL) | 59.6 (13.4) | 58.4 (13.6) | NS |
| LDL-cholesterol (mg/dL) | 106.9 (24.5) | 110.5 (26.7) | 0.016 |
| HDL indicates high-density lipoprotein cholesterol; LDL (low-density lipoprotein cholesterol); NS- non significant. Values are expressed as mean (standard deviation). | |||
Table 2: Mean plasma lipid and glucose level variations in obese children as per sex.
Discussion
It is well understood by all pediatricians that the cardiovascular risk factors in obese children appearing early in life will continue lifelong. These will also worsen if primary prevention is not started early in children with a high BMI. This cluster of cardiovascular risk factors will subsequently lead to morbidity and mortality in even young adults. To track individuals with risk of excess weight and cardiovascular risk factors, anthropometry is considered a very useful method. Measuring of anthropometry is not expensive and can be universally applied.
There are several studies related to cardiovascular risk factors in adults. But there are few studies dealing with the cardiovascular risk factors in children. There have been a few earlier studies. [22,23]. which have shown the association between various risk factors and atherosclerosis in pre pubertal children. In our study, we observed a strong association between the BMI percentile and cardiovascular risk factors such as increased blood pressure, low HDL-cholesterol and increased TG levels in pre pubertal children.
Obese children are many times more likely to have hypertension than non-obese children. Accurate BP assessment is very essential. However, ambulatory BP monitoring is believed to be more accurate than spot clinical BP monitoring because it monitors BP changes more precisely that occur throughout the day. Ambulatory BP monitoring is also considered better because regular office BP measurements may occasionally miss it. Components of the metabolic syndrome (dyslipidaemia, hypertension, hyperinsulinism, elevated blood glucose level and elevated ALT) have been shown to cluster in overweight/ obese children as well as adults. This clustering of risk factors is known to be associated with increased long-term morbidity and mortality in adults. Further longitudinal studies are needed to assess these in children.
Hypertension, high total cholesterol, low HDL, high LDL and high TG are recognized as risk factors for cardiovascular disease in adults as well as children. In our study, obese children had higher systolic and diastolic blood pressure than normal weight children, by 8.5 mm Hg and 4.7 mm Hg, respectively. Therefore, if these high blood pressures are allowed to track unchecked into adulthood, obese children could have a 30-40% higher risk of future stroke and ischaemic heart disease than their normal weight counterparts.
In children, low HDL and high TG maybe more strongly related to BMI. Like in our study, some other studies also found a positive relationship with increasing BMI z-score for these measures. The linear relationship between increasing BMI and blood pressure like in our study was comparable to other studies. [24-26]. Our results revealed that with the exception of FBG and TC, overweight/obese children had significantly higher anthropometric and biochemical indices compared to normal-weight counterparts. Authors of an Indian study have suggested that high FBG may only be visible when other metabolic components start appearing. [27]. Thus, it may take many years for FBG to be visibly high among many overweight/ obese children.
Childhood obesity is becoming increasingly prevalent around the world. Study by Ciconne et al. has shown that overweight or obese children have an initial endothelial dysfunction and vascular damage which is the first stage in the development of atherosclerosis. [28]. In another study [29]. by the same author, he has said that various coagulation and metabolic parameters like high molecular weight adiponectin is related to cardiovascular risk in overweight/obese children.
Our observations, combined with those of other researchers, suggest that failure to reverse this trend may have wide-reaching consequences on the quality and longevity of life of young children. Such evidence stresses the importance of preventing obesity starting in the early years of life. Thus control of obesity and cardiovascular risk factors could give them a head start on their normal and even over weight class mates for future cardiovascular disease, diabetes, and stroke. Therefore, the effect of obesity on future health, particularly cardiovascular health, could be far greater than previously thought of.
There are different methods available for determining body composition in children like body adiposity index (BAI), dualenergy X-ray absorptiometry (DEXA), computed tomography (CT) and magnetic resonance imaging (MRI). CT and MRI are being recently used as adiposity phenotyping reference methods. However, their use is complicated by the absence of a gold standard and the lack of validity data in children. [30]. As we were doing the study in basic field conditions, CT and MRI were not used. Besides, CT also had the disadvantage of radiation exposure besides the cost factor.
Besides, BMI, skin-fold thickness measurements are considered good indicators because they are a direct measure of the fat layer. However, the measurements are site and sex-specific. [31]. One of the limitations of SFT is that visceral fat (fat in the abdominal cavity) is not measured. There also can be considerable variability across practitioners, leading to the requirement for specific training. Besides being time consuming, measurement error, technical expertise and subject compliance are limitations of this method. We also do not have standardized reference data for skin fold thickness in children as we have for BMI. [32].
The statistical relationships between skin-folds and percent or total body fat in children and adults are often not as strong as that of BMI. [33]. We used BMI as a measure of obesity because of the ease of collecting data from the OPD population. Besides, it is the most commonly used anthropometric measure for obesity in children and there are several reference data sets available.
Limitations
There were certain limitations in our study.
Firstly, there was a high level of heterogeneity in our study population. Although we defined inclusion criteria carefully to ensure the homogeneity of study population but factors such as ethnicity, pubertal status and age still varied.
Secondly, another limitation of our study was the bias of researchers. The researchers analyzing the biochemical parameter levels were frequently not blinded to the BMI of the child. However, the risk of bias from imprecise or incorrect measurement methods was kept as low as possible.
Thirdly, we were unable to compare the influence of age and pubertal status on obese and non-obese children because very few papers reported data on these details. This lack of detail suggests a need for more primary research into the effect of weight on cardiovascular disease risk in different age groups.
Conclusion
The conclusions derived from our study only provided an indirect hint of the risk of cardiovascular diseases in children at the time of measurement of all parameters. We could not establish the relationship between risk parameters for cardiovascular disease and the ongoing changes in weight of the same child. Also we could not predict how the cardiovascular disease risk factors in these children would progress into adulthood.
Acknowledgement
We express our sincere thanks to the Department of Biochemistry, Base Hospital, Delhi Cantonment for all the support in making this study successful.
Conflict of Interest
All authors disclose that there was no conflict of interest..
REFERENCES
- World Health Organization. Global prevalence and secular trends in obesity Preventing and managing the global epidemic. Consultation on obesity. World Health Organization, Geneva. 1998. p. 17-40.
- Holst-Schumacher I, Nuñez-Rivas H, Monge-Rojas R, Barrantes-Santamaría M. Components of the metabolic syndrome among a sample of overweight and obese Costa Rican schoolchildren. Food Nutr Bull 2009, 30:161-70.
- Yoshinaga M, Tanaka S, Shimaga A, Sameshima K, Nishi J, Nomura Y, et al. Metabolic syndrome in overweight and obese Japanese children. Obes Res 2005, 13:1135-1140.
- Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. PediatrObes. 2012;7:284-294.
- Khadilkar V, Yadav S, Agrawal KK, Tamboli S, Banerjee M, Cherian A. et al. Revised IAP Growth Charts for Height, Weight and Body Mass Index for 5- to 18-year-old Indian Children. Indian Pediatr 2015;52: 47-55
- Garnett SP, Baur LA, Srinivasan S, Lee JW, Cowell CT. Body mass index and waist circumference in midchildhood and adverse cardiovascular disease risk clustering in adolescence. Am J ClinNutr 2007, 86:549-555.
- Sung RY, So HK, Choi KC, Nelson EA, Lim AM, Yin JA, et al. Waist circumference and waist to height ratio of Hong Kong Chinese children. BMC Public Health 2008, 8:324.
- IDF: The IDF Consensus Worldwide Definition of the Metabolic Syndrome in children and adolescents. International Diabetes Federation, Belgium, 2007.
- Ribeiro JC, Guerra S, Oliveira J, Teixeira-Pinto A, Twisk JW, Duarte JA, et al. Physical activity and biological risk factors clustering in pediatric population. Prev Med 2004;39:596-601.
- Steene-Johannessen J, Kolle E, Anderssen SA, Andersen LB. Cardiovascular disease risk factors in a population-based sample of Norwegian children and adolescents. Scand J Clin Lab Invest 2009;69:380-386.
- Giannini C, DeGiorgis T, Scarinci A, Cataldo I, Marcovecchio ML, Chiarelli F, et al. Increased carotid intima-media thickness in pre-pubertal children with constitutional leanness and severe obesity: The speculative role of insulin sensitivity, oxidant status, and chronic inflammation. Eur J Endocrinol 2009;161:73-80.
- Graf C, Rost SV, Koch B, Heinen S, Falkowski G, Dordel S. Data from the STEP TWO programme showing the effect on blood pressure and different parameters for obesity in overweight and obese primary school children. Cardiol Young 2005;15:291-298.
- Reinehr T, Schaefer A, Winkel K, Finne E, Toschke AM, Kolip P. An effective lifestyle intervention in overweight children: findings from a randomized controlled trial on“Obeldicks light.”ClinNutr 2010;29:331-336.
- Mirzaei M, Taylor R, Morrell S, Leeder SR. Predictors of blood pressure in a cohort of school-aged children. Eur J CardiovascPrevRehabil 2007;14:624-629.
- Savva SC, Tornaritis M, Savva ME, Kourides Y, Panagi A, Silikiotou N,et al. Waist circumference and waist-to-height ratio are better predictors of cardiovascular disease risk factors in children than body mass index.Int J Obes 2000;24:1453-1458.
- Thomas NE, Cooper SM, Williams SP, Baker JS, Davies B. Relationship of fitness, fatness, and coronary-heart-disease risk factors in 12-to-13-year olds.PediatrExercSci 2007;19:93-101.
- Vizcaino VM, Aguilar FS, Martinez MS, Lopez MS, Gutierrez RF, Rodriguez-Artalejo F. Association of adiposity measures with blood lipids and blood pressure in children aged 8-11years.ActaPaediatr 2007;96:1338-1342.
- Teixeira PJ, Sardinha LB, Going SB, Lohman TG. Total and regional fat and serum cardiovascular disease risk factors in lean and obese children and adolescents. Obesity 2001;9:432-442.
- Woo KS, Chook P, Yu CW, Sung RYT, Qiao M, Leung SSF, et al. Effects of diet and exercise on obesity-related vascular dysfunction in children.Circulation2004a;109:1981-1986.
- Andersen LB, Wedderkopp N, Hansen HS, Cooper AR, Froberg K. Biological cardiovascular risk factors cluster in Danish children and adolescents: the European Youth Heart Study. Prev Med 2003;37:363-367.
- Jago R, Harrell JS, Mc Murray RG, Edelstein S, El-Ghormli L, Bassin S. Prevalence of abnormal lipid and blood pressure values among an ethnically diverse population of eighth-grade adolescents and screening implications.Pediatrics. 2006;117:2065-2073.
- Freedman DS. Childhood obesity and coronary heart disease. PediatrAdolesc Med. 2004;9:160
- McCrindle B. Will childhood obesity lead to an epidemic of premature cardiovascular disease? Evid Based Cardiovasc Med. 2006;10:71.
- Bell LM, Byrne S, Thompson A, Ratnam N, Blair E, Bulsara M, et al. Increasing body mass index z-score is continuously associated with complications of overweight in children, even in the healthy weight range. J ClinEndocrinolMetab. 2007;92:517-522.
- Lobstein T, Baur LA, Uauy R. Obesity in children and young people: A crisis in public health. Obes Rev. 2004; Suppl 1:4-104.
- Sorof JM, Urbina EM, Cunningham RJ, Hogg RJ, Moxey-Mims M, Eissa MA, et al. Screening for eligibility in the study of antihypertensive medication in children: experience from the Ziac Pediatric Hypertension Study. Am J Hypertens. 2001; 14: 783-787.
- Misra A, Vikram NK. Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications. Nutrition. 2004;20:482-491.
- Ciccone MM, Miniello V, Marchioli R, Scicchitano P, Cortese F, Palumbo V.Morphological and functional vascular changes induced by childhood obesity. Eur J CardiovascPrevRehabil2011;18;831-835.
- Ciccone MM, Faienza MF, Altomare M, Nacci C, Montagnani M, Valente F, et al. Endothelial and Metabolic Function Interactions in Overweight/Obese Children. J AtherosclerThromb. 2016;23:950-959.
- Wells JCK,Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91:612-617.
- Livingstone B. Epidemiology of childhood obesity in Europe. Eur J Pediatr. 2000;59:14-34.
- Freedman DS, Katzmarzyk PT, Dietz WH, Srinivasan SR, Berenson GS. Relation of body mass index and skinfold thickness to cardiovascular disease risk factors in children: the Bogalusa Heart Study. Am J ClinNutr. 2009;90: 210-216.
- Roche AF, Siervogel RM, Chumlea WC, Webb P. Grading body fatness from limited anthropometric data. Am J ClinNutr. 1981;34:2831-2838.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.