Corticosteroid Intervention in Management of Coronavirus COVID 19 Infection A Systematic Review and Meta Analysis
2 Program Director, Dental Public Health, Riyadh Elm University, Riyadh, Saudi Arabia, Email: naviningle123@gmail.com
3 Associate Professor, Department of Prosthodontics, Vice President Rector for Post Graduate & Scientific Research, Riyadh Elm University, Riyadh, Saudi Arabia
4 Assistant Professor, Dental Public Health, Riyadh Elm University, Riyadh, Saudi Arabia, Email: abdulprevent8@gmail.com
Citation: AIbakr AA, et al.: Corticosteroid Intervention in Management of Coronavirus COVID 19 Infection A Systematic Review and Meta Analysis. Ann Med Health Sci Res. 2020;10:1163-1167.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction & Aim: The coronavirus disease is very intense and widely spreading across the world. Different treatment modalities are unsuccessful till date. Many researchers are still finding out the drug to cure COVID – 19 diseases. This review is to assess corticosteroid intervention in COVID-19 patients for outcomes of mortality and morbidity. Methods: Literature search of published articles in Medline, Scopus, Ovid and Journal of Web till June 2020 were reviewed for the pre-described outcomes. RevMan 5.4 software was used for analysis of study. Results: Of the 186 articles chosen, 6 were chosen for qualitative analysis and 3 for quantitative analysis. Meta analytic comparison showed that mortality rate was higher in patients treated with corticosteroids (OR = 0.98, 95% CI = 0.72 – 1.34, p<0.0001) than those without. 337 patients died out of 1389 corticosteroid managed COVID -19 patients. Conclusion: Corticosteroid usage increases the odds of increased mortality among COVID -19 patients.
Keywords
Corticosteroids; Coronavirus; COVID -19; Mortality; Respiratory failure
Introduction
Corona virus or COVID-19 is novel single stranded RNA virus exhibiting diverse characteristics, resulting in a plethora of symptoms such as fever, fatigue, dry cough, muscle fatigue and breathing difficulties. [1] Coronavirus pandemic surfaced in Wuhan, Republic of China in 2019, has engulfed mankind in grave suffering and losses. [2] Literature evidence supports the respiratory transmission of Corona virus through coughing and sneezing for human to human spread. [3,4]
As of May 2020, the numbers of COVID-19 affected cases were 9.1 million confirmed cases while causalities reported to be 472000 deaths. [5] The initial symptoms of COVID-19 are cough and fever, with onset of dyspnoea in 20% of the cases, and with pulmonary infiltration in 10%. Around 25% of the hospital admitted cases develop Acute Respiratory Distress syndrome (ARDS) within 10.5 days following onset of symptoms. [6] It is hypothesized that corticosteroids when administered to COVID-19 patients, the risk of developing ARDS reduced due to inflammatory response modulation. Unfortunately, neither a vaccine nor a therapy is devised against this virus. Hence it is imperative to identify and explore the drug therapy intervention to tackle this global issue. [7] Corticosteroids have demonstrated appreciable inhibitory effect on factors of inflammation and form the preferred auxiliary therapy in case of viral pneumonia. [8] Inhaled corticosteroids have shown beneficial effect against coronavirus infection. Human respiratory epithelial cells when treated in-vitro with budesonide and a combination of glycopyrronium and formoterol exhibited inhibitory effect on coronavirus HCoV-229E duplication and production of cytokine. [9]
Yet no conclusive evidence is derived regarding the role of corticosteroids in COVID-19 infections. Hence, this systematic review and meta-analysis was conducted to explore the effect of corticosteroids on Corona virus infection answering the research question ““What will be the effect or outcome of corticosteroids on Coronavirus infection?” This review and meta-analysis was done to identify the role of corticosteroids on coronavirus infection and to compare the mortality and morbidity rate of steroid intervened corona virus infected patients.
Literature Search
Protocol and Registration
The PRISMA checklist, used for reporting systematic reviews and meta-analysis was employed for this analysis.
Eligibility criteria
The research question was focused using the “PICOS” framework. The research question formulated used to determine the inclusion and exclusion criteria.
Population: Patients with coronavirus infection (COVID-19).
Intervention: Corticosteroid administration, in any form (Intravenous or inhalational).
Comparison: Population free from coronavirus infection formed the comparison group.
Outcome: The primary outcome assessed was mortality. Secondary outcomes of length of stay and complications were also taken into considerations wherever mentioned.
Setting: Private practice or hospital or cases reported in normal population.
Inclusion criteria: Studies assessing the outcome of corticosteroid treated patients with coronavirus infection.
Exclusion criteria: No evaluation of editorials, case reports and commentaries and articles written in language other than English were made.
Information sources
Search engines like MEDLINE, Ovid, Scopus and Journal on web databases were searched for literature. All searches were performed through EBSCO. All relevant articles identified, which were obtained in full, via electronic and other search methods were checked. Abstract and conference proceedings were used to search and identify unpublished studies.
Search strategy
Keywords: Key terms were used for the search: (1) Coronavirus; ( 2) COVID-19; (3) SARS; (4) corticosteroid; (5) steroid; (6) severe acute respiratory syndrome; (7) prednisolones and (8) dextamethoasone.
Boolean operators: The Boolean operator ‘OR’ was employed to complement truncated synonyms in each search theme. The Boolean operator ‘AND’ makes up the sum of each four main search themes to specifically output papers which give at least one result for each time.
Search limits: Searches incorporated literature from the year 2000 up until 2020 as the concluding year for the search. Only sources in English were used.
Process of study identification: Endnote X8 was used to import the results of the search data and to remove the duplicates. The screening of abstracts will be carried out by the use of the eligibility criteria and for those not excluded; full text articles were searched for. These were, then, assessed for inclusion and upon acceptance, underwent data extraction and quality assessment. Articles, failing to meet inclusion criteria, were omitted.
Data collection
All the title and the extracts were independently screened by the reviewer and upon a meticulous review of the full text articles, the data were extracted and documented in a data extraction table, which shows depicting data items evaluated for the review.
Data items
The data extraction table will include Study ID, age, sample size, study population, type of corticosteroid, study design and outcome.
Risk of bias in individual studies
The New Castle Ottawa scale (NOS) was be used for quality assessment, which will include patient selection, study comparability and outcome assessment.
Data synthesis
Out of the 6 articles reviewed, 3 articles were processed for data synthesis as the rest did not provide data on comparison group.
Statistical analysis
Data analysis was carried out using RevMan 5.4 software.
Results
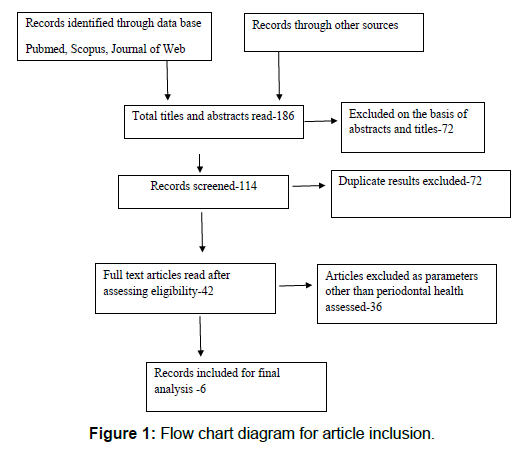
The search strategy yielded a total of 186 articles, out of which 72 were removed because of duplication. Further 72 articles had to be excluded as only abstracts were obtained of these articles. A total of 6 articles were included for the systematic review and only 3 were analyzed for meta-analysis as the rest did not mention comparison group [Figure 1].
The review had 6 articles. Out of which 5 studies were retrospective observational studies or case series and one was a prospective observational study.
This review included 9 articles, all of which were randomized, parallel design controlled trial used both for systematic review and meta-analysis. All studies were done on genders, males and females. Hydrocortisone and methylprednisolone were the most commonly used steroid types. The study characteristics are summarized in Table 1. [10-14]
| Study ID | Sample | Age | Location | Population | Corticosteroid intervention | Outcome | Study design |
|---|---|---|---|---|---|---|---|
| Arabi et al. [10] | 309 with 151 patients treated with corticosteroids | 57.2 + 17.2 | Saudi Arabia, 14 tertiary care hospitals | Critically ill patients infected with Middle East Respiratory syndrome |
Systemic corticosteroids (Hydrocortisone – equivalent doses { methy prednisolone 1:5; dexamethasone 1:25; prednisolone 1:4} |
Subjects receiving corticosteroid treatment had a higher crude 90 day mortality (74.2%) when compared to controls (57.6%); longer length of stay in ICU (median [Q1–Q3], 12.5 d [8.0–23.0 d] compared with 7.0 d [5.0–13.0 d]; P,0.0001) and extended hospital stay median [Q1–Q3], 21.0 d [13.0–38.0 d] compared with 15.0 d [8.0–30.0 d]; P = 0.0006 |
Retrospective Observational study. |
| Chen et al. [4] | 401 patients | 34.74 + 13.31 | Guangzhou, China | SARS affected patients confirmed with a fourfold rise of SARS – IgG during 10 -14 days onset and a positive polymerase chan reaction test in the acute stage | Methyprednisolone majorly and hydro prednisone or dextamethoasone in lesser amounts | After adjusting for confounding variables of corticosteroid usage, age, rigor at onset and complications, corticosteroid therapy presented reduced mortality and shorter stay in hospital. (p<0.05) | Retrospective Observational study |
| Yang et al. [11] | 15 patients | Median of 72 (62 – 74) | Jewish General hospital, Montreal, Canada | Critically ill COVID 19 Patients with cytokinase release syndrome (CRS)presenting with hypoxic respiratory failure, vasoplegic shock on multiple vasopressors or both respiratory and cardiovascular failure | 9 patients were treated with methylprednisolone; 4 cases on hydrocortisone and 2 on dextamethasone | A significant clinical and biochemical correlation was demonstrated for steroid intervention and enhanced surrogate outcome in delayed onset CRS associated with COVID 19 | Retrospective Case series |
| Yam et al. [12] | 1188 patients treated with corticosteroids and 99 controls | Hong Kong, 12 hospitals | SARS patients who were above 18 years and on corticosteroids for lesser than 14 days from the onset of symptoms | Intravenous Hydrocortisone; Intravenous methyprednisolone; Oral Prednisolones and Intravenous pulsed corticosteroid | Crude death rate was reported to be lesser in steroid intervened cases as compared to controls (17.0% vs 28.3%). Of the four groups of steroids, mortality rate was lesser in low dose oral prednisolone and high dose methylprednisolone | Retrospective Observational study | |
| Huang C [3] | 41 patients | 49 (41 – 58) | Huanan province | Laboratory confirmed 2019 n CoV disease via real time RT – PCR and next generation sequencing | Methylprednisolone 40 – 120 mg per day | All patients had pneumonia. 13 were admitted to ICU as they showed high flow nasal cannula or greater level oxygen support measures to overcome oxygen deficiency | Prospective study |
| Wang et al. [13] | 138 hospitalized cases with Novel Coronavirus infected patients | 56 years median (Interquartile range = 42 – 68 years | Zhongnan Hospital, Wuhan, Hubei Province | NCIP patients with severe form receiving intensive care unit facilities and the non – so severe cases without ICU care | Not mentioned | The ICU NCIP cases presented with a higher prevalence of pharyngeal pain, dyspnea, dizziness, abdominal pain and anorexia. Complications included shock, arrhythmia, ARDS and acute heart failure | Case series |
Table 1: Characteristics of studies included.
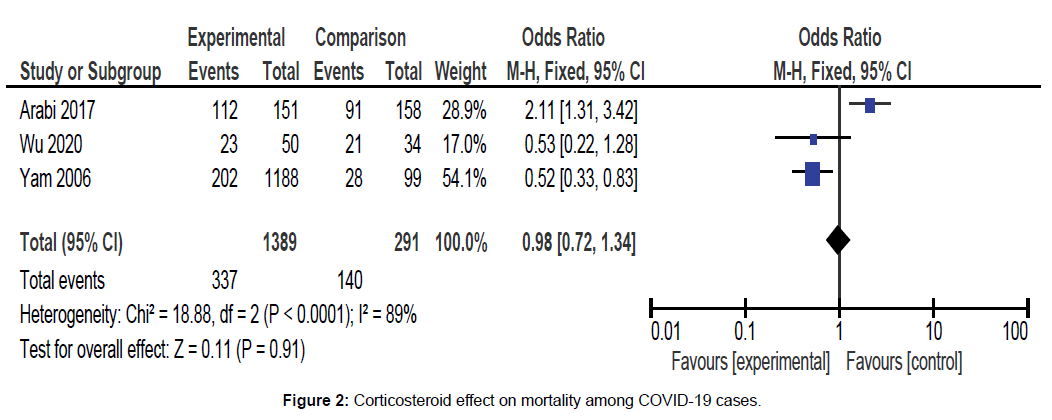
Meta analytic results showed that no significant difference was noted between cases that were treated with corticosteroids as compared to those without being intervened with corticosteroids for mortality rate. (OR = 0.98, 95% CI = 0.72–1.34, p=0.91), the random effects model was adopted. Out of the total 1389 corticosteroid treated COVID-19 cases, 337 of them died. [Figure 2].
Five studies assessed number of COVID-19 cases turning to complications during steroid therapy. Of 1434 patients intervened with steroids, 227 developed to complications accounting to 15.82% [Table 2].
| Study ID | Complications | Total number |
|---|---|---|
| Chen RC et al. | 89 | 152 |
| Yam et al. | 49 | 1088 |
| Wang et al. | 72 | 138 |
| Yang et al. | 4 | 15 |
| Huang et al. | 13 | 41 |
| Total | 227 | 1434 |
Table 2: COVID-19 cases progressing to complications following steroid interventions.
Discussion
The current review analysed the morbidity rate of COVID-19 affected cases treated with corticosteroids and those who were not on steroid therapy. COVID-19 infections are spreading widely as a pandemic affecting various nations. Unfortunately, no an effective antiviral therapy exists against this virus while efforts are made to treat this infection in symptomatic fashion. Steroids are the usual choice of drugs in case of pneumonia or acute respiratory failure enticing these cases. Yet, no conclusive evidence is arrived at for the usage of corticosteroids in corona virus infection. While Russell et al. [15] contradicts corticosteroids recommendation in SARS-CoV-2-induced lung injury or shock, Chinese physicians [15] support the use of low to moderate dose corticosteroids, for Corona virus patients critically affected with pneumonia.
The present review analyzed that COVID-19 infected cases on steroid intervention reported a higher mortality rate and morbidity occurrence. This could be possibly explained on an hypothesis which showed that glucocorticoids inhibits IL–2 and interferon- γ (IFN- γ) production in T lymphocytes. The focus moves to Th2 T cell response from Th1 cell response, inducing programmed cell death amongst various immunologically relevant cells, with inclusion of immature T and B cell predecessors and T cells of mature type. [16] Another hypothesis proposes the association of already existing CD4 T cells with reduced viral deprivation and reduced severity of infection. [17] Literature evidence shows corticosteroids usage can result in viral RNA removal in long term from airways, blood and faeces thus increasing the mortality risk, co morbidity conditions and greater length of stay. [18,19]
Also, corticosteroid therapy is proposed usually in hypoxemic patients and those exhibiting moderate grade of positive end expiratory pressure, stressing that steroids are initiated in critically ill patients, cases with comorbid conditions and those not presenting any signs of improvement. [20] This marks for the higher mortality cases in steroid managed group.
In the study of Huang C et al. [3] all patients (13 cases) developed pneumonia. Pneumonia forms the major complication of Corona virus infection. This study also presented a rapid death rate, with 15% (6) of 41 patients succumbing to ARDS within two days after hospital admission.
The study of Yang et al. [11] reported 15 COVID-19 affected severe cases admitted to ICU, all with Cytokinase Release Syndrome (CRS). It has presenting symptoms of fever, ARDS, multiple organ failure and hemodynamic collapse because of shock. But late onset cases will only worsen the condition as is the case by delayed onset of systemic inflammatory response for cytokine irregulation termed as CRS. Patients become hypoxic or vasoplegic due to increase in C- reactive protein or interleukin–6, with no viral symptoms. In these conditions, corticosteroids form the main line of therapy, and naturally present lesser prognostic rate.
The study results must be interpreted with caution as 5 out of 6 were retrospective studies as they invoke selection bias and also has a low level of evidence. Randomized control study design is more appropriate to tackle selection bias and confounding effect. But none of the studies reported had this study design. In future, RCT done in multiple centers are recommended to better understand the intervention process and effect of steroids in COVID-19 patients. Also, the corticosteroid administered in these patients did not conform to any uniform dosage nor timing which could have had an influence in the effect assessed.
Conclusion
Corticosteroids are recommended in severe cases of COVID-19 infections. Steroid intervention in coronavirus patients presents a high mortality rate, adverse effects and increased length of stay.
Competing Interests
The authors declare that they have no competing interests.
Authors Contributions
AAA and NAI conceived the proposal; AAA, MKA and MAB collected the data, participated in data analysis and interpretation; NAI, AAA, and MKA supervised data quality, participated in data analysis, interpretation and drafted the manuscript; MAB, AAA and NAI finalized the manuscript. All the authors have read and agreed to the final manuscript.
REFERENCES
- He F, Deng Y, Li W. Coronavirus disease 2019 (COVID-19): what we know? J Med Virol. 2020;92:719-725.
- Koczkodaj WW, Mansournia MA, Pedrycz W, Wolny-Dominiak A, Zabrodskii PF, Strzaška D, et al. 1000,000 cases of COVID-19 outside of China: the date predicted by a simple heuristic. Glob Epidemiol. 2020;2:100023.
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England). 2020;395:497-506.
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet (London, England). 2020;395:514-523.
- News.google.com/covid19/maphl. Accessed on 23 June 2020.
- Halpin DMG, Singh D, Hadfield RM. Inhaled corticosteroids and COVID-19: a systematic review and clinical perspective. Eur Respir J. 2020;55:2001009.
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci Trends. 2020;16:69-71.
- Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-in- flammatory effects of glucocorticoids. NeuroImmuno Modul. 2015;22:20-32.
- Yamaya M, Nishimura H, Deng X, Sugawara M, Watanabe O, Nomura K, et al. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir Investig. 2020;58:155-168.
- Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med. 2018;197:757-767.
- Stephen SY, Lipes J. Corticosteroids for critically ill COVID-19 patients with cytokine release syndrome: a limited case series. Can J Anesth. 2020;67:1462-1464.
- Yam LY-C, Lau AC-W, Lai FY-L, Shung E, Chan J, Wong V. Corticosteroid treatment of severe acute respiratory syndrome in Hong Kong. J Infect. 2007;54:28-39.
- Wang Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, P.R China. JAMA. 2020:e201585.
- Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet (London, England). 2020;395:473-475.
- Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-n- CoV pneumonia. Lancet (London, England) 2020;395:683-684.
- Gonzalo JA, González-García A, Martínez C, Kroemer G. Glucocorticoid-mediated control of the activation and clonal deletion of peripheral T cells in vivo. J Exp Med. 1993;177:1239-1246.
- Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4 + T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;29:274-280.
- Lee N, Allen Chan KC, Hui DS, Ng EKO, Wu A, Chiu RWK, et al. Effects of early corticosteroid treatment on plasma SARS-associated coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304-309.
- Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl). 2020;133:1039-1043.
- Delaney JW, Pinto R, Long J, Lamontagne F, Adhikari NK, Kumar A, et al. Canadian Critical Care Trials Group H1N1 Collaborative. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care. 2016;20:75.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.