COVID-19 Comorbidities and Complications Leading to Mortality
2 Department of General Medicine, Amrita Institute of Medical Sciences and Research Centre, Kochi, Kerela, India
3 Department of Bio Statistics, Amrita Institute of Medical Sciences Data Analysis, Kochi, Kerela, India
4 Department of Internal Medicine, DY Patil Medical College, Pune, Mumbai, India
5 Department of Internal Medicine, Amrita Institute of Medical Sciences, Kochi, Kerela, India
Received: 06-Jan-2023, Manuscript No. amhsr-23-85637; Editor assigned: 09-Jan-2023, Pre QC No. amhsr-23-85637 (PQ); Reviewed: 27-Jan-2023 QC No. amhsr-23-85637; Revised: 03-Feb-2023, Manuscript No. amhsr-23-85637 (R); Published: 10-Feb-2023
Citation: Smitha K, et al. COVID-19 Comorbidities and Complications Leading to Mortality. Ann Med Health Sci Res. 2023;13:407-412.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
472 corona virus infected cases admitted to Amrita hospital from 2020 to 2022 were studied retrospectively and prospectively. Primary aim was to identify the risk factors, to predict the prognostic factors of mortality and assess the association of inflammatory markers with complications in COVID infection. Based on the proportion (16.9%) of risk factor(hypertension) of COVID 19 Patients with 20% relative precision and 95% confidence the minimum sample size comes to 472. SPSS-Software with frequency for Qualitative data, Independent Sample T test and logistical regression were used. Overall Disease Free Survival, Kaplan Meier analysis will be applied along with log rank test. To test the statistical significance of the difference in the proportion of inflammatory markers with the complications, Chi square test will be applied. Diabetes Mellitus, Coronary artery Disease, Chronic Liver Disease and malignancy contributed significantly to mortality. Age more than 60 years, male sex, median D-dimer, NLR ratio, low platelet count and high creatinine values showed statistically significant difference in the patients who died. The complications, hyponatremia were seen in (17%, ORR-3.11), Pneumonia in (70(14.8%)) Sepsis in (6.6% (31)) and UTI in (8.1% (38)). The mortality was 34.3% ( OR15.57) in pneumonia, and 61.3% in sepsis (OR 37.12 in univariate and 12.42 in multivariate analysis), 15.2% in UTI. CRP (OR-1.005), D-dimer (OR-1.009) and ferritin (OR-1) in multivariate analysis showed significance in pneumonia. High Serum ferritin (OR-1), low platelet count (OR 0.991) and elderly (OR-1.002) were independent predictors of mortality in sepsis related to COVID.
Keywords
COVID- 19; Co-morbidities; Sepsis; Pneumonia; Mortality
Introduction
COVID-19 infection generally run a mild course in up to 80% of those affected. A number of pre-existing co-morbidities determine the severity of infection and the outcome in an individual patient[1].
The most important of these co-morbidities are pre-existing diabetes, obesity, hypertension, chronic lung disease and malignancy. This comprehensive review discusses the impact of these co-morbidities and the role of laboratory predictors of poor patient outcomes. Whether early identification of co morbidities or lab parameters can avert serious outcomes
Aim
Primary
Identify the risk factors in patients with COVID infection
Secondary
• To predict the prognostic factors of mortality in COVID patients.
• To assess the association of inflammatory markers with complications.
Study design
Retrospective and prospective observational study.
Study setting
Patients admitted with COVID 19 infection in AMRITA hospital, Kochi, Kerala, India
Inclusion criteria
• Age>18 years
• Patients with proven COVID infection
• COVID antigen/RTPCR/True nat/GENExpert Positive
Based on the proportion (16.9%) of risk factor(hypertension) of COVID 19 Patients with 20% relative precision and 95% confidence the minimum sample size comes to 472 [2].
Methodology
• Retrospective and prospective study observational study.
• COVID patients admitted from January 2020 will be analysed in terms of Co morbidities, Hospital stay, complications and lab parameters.
Sample size
Based on the proportion (16.9%) of risk factor (hypertension) of COVID 19 Patients with 20% relative precision and 95% confidence the minimum sample size comes to 472.
Parameters assessed
• Age, Sex, NLR ratio, CRP, Ferritin, D-dimer, Duration of hospital stay, Pre-existing DM, HTN CAD, COPD, hypothyroidism, CVA, Cancers, CKD, bronchial asthma and CLD
• Complications like pneumonia UTI Hyponatremia and sepsis.
Statistical details
SPSS-Software is used for analysis of data. Frequency charts are used for Qualitative data. Independent Sample T test and logistical regression will be used. To find the survival probability of Overall Survival/Disease Free Survival in COVID 19 patients, Kaplan Meier analysis will be applied along with log rank test. To test the statistical significance of the difference in the proportion of inflammatory markers with the complications in COVID 19 Patients, Chi square test will be applied.
Results
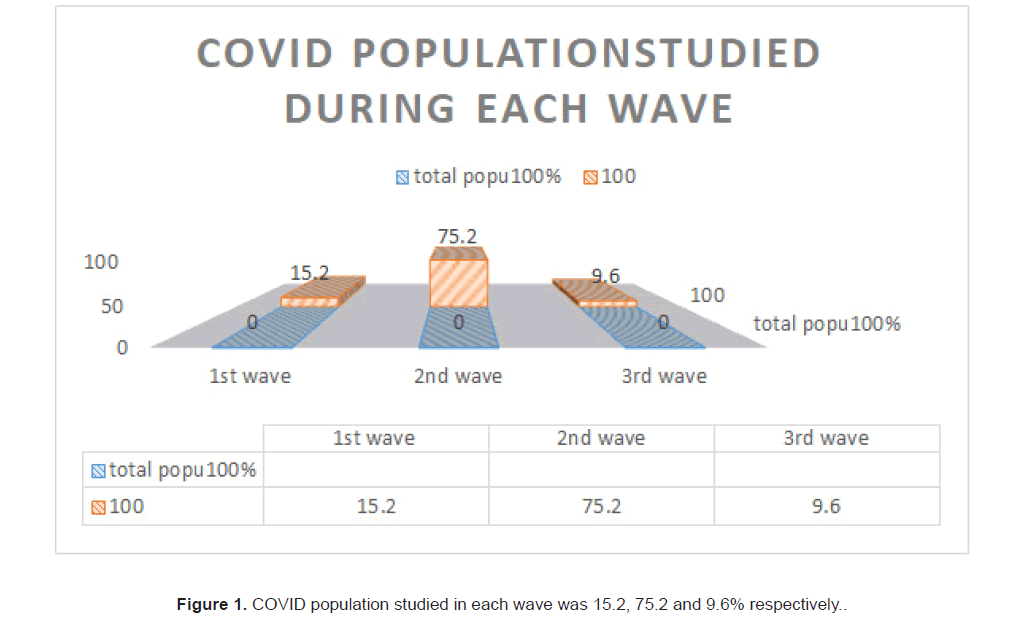
472 corona virus infected cases admitted to Amrita hospital during the period of 2020 to 2022 were studied retrospectively and prospectively. All patients had either COVID rapid antigen or COVID RTPCR positive. Of the 472 cases, 15.25% (72) developed their infection during the first wave, 75.21% (355) patients belonged to end of first wave and the second wave, caused by the delta variant of COVID and 9.5% cases were studied during 2022, during the third wave caused by the omicron variant (Figure 1).
Demographics
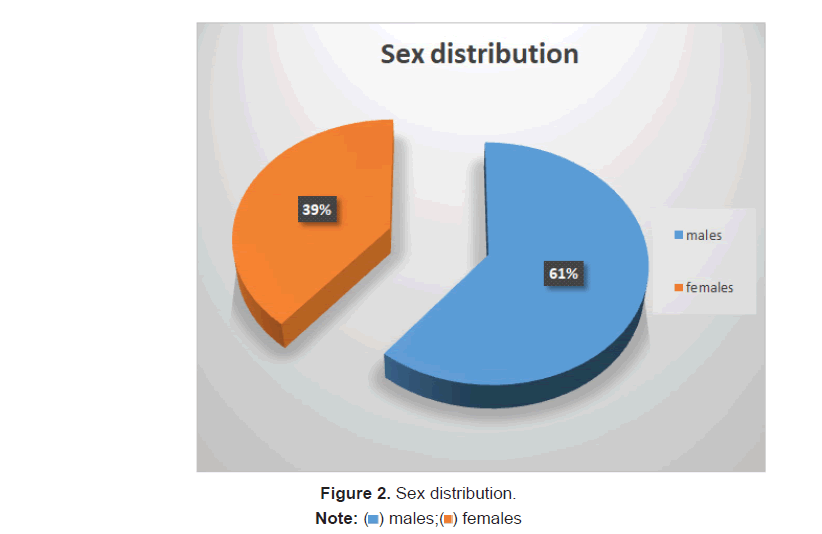
Mortality was 7.8%(37) in the study.Mean age of the patient who died was 64.6 ± 1.59 years and the alive patients had a mean age of 53.94 ± 17.28. 61.7% (291) were males and 38.3% (181) were females. 8.9% of Males and 6.1% of ladies died (Figure 2).
Laboratory values and outcome
Median CRP values of 46.2 (25, 115.08) was seen in patients who died whereas it was low, 9.1 (2.72, 34.46) in the patients who survived. The median D-dimer was 2.1 microgram/ml (1.07, 7.16) in the patients who succumbed to the disease. The median D- dimer in the alive group was 0.52 mcg/ml (0.32, 1.15) only. The median ferritin levels in patient who died were 659.3 (353, 1290) as against 203 (85.5, 449.85) in patients who survived. The median WBC count was elevated (9.060) (6.12, 15.93) in patients who died and was lower i.e 6.625 (5.12, 8.42) in patient who survived. NLR ratio was significantly high 10.2 (4.28, 19.6) in patients who died. Median platelet count was 184 (114.5, 234) among who died and 227 (179, 285) in those who were alive. Median creatinine was 1.16(0.82, 2.42) among the patients who died and 0.9(0.735, 1.22) among alive patients (Table 1).
| Variables | Outcome | |||||
|---|---|---|---|---|---|---|
| Alive | Death | Univariate OR | p value | Multivariate OR | ||
| Age | 53.94±17.28 | 64.65±1.59 | 1.043 | <0.001 | 1.04 | |
| CRP | 9.1(2.72,34.46) | 46.2(25,115.08) | 1.008 | <0.001 | ||
| D-dimer | 0.52(0.32,1.15) | 2.1(1.07,7.16) | 1.002 | <0.001 | ||
| Ferritin | 203.3(85.5,449.85) | 659.3(353,1290) | 1.001 | <0.001 | 1 | |
| WBC count | 6.62(5.12,8.42) | 9.06(6.12,15.93) | 1 | <0.001 | ||
| Neutrophil count | 65.5(55.7, 76.4) | 85.9(74.95.91) | 1.08 | <0.001 | 1.07 | |
| NLR ratio | 2.93(1.76,5.15) | 10.2(4.28,19.6) | 1.02 | <0.001 | ||
| Platelet | 227(179,285) | 184(114.5,234) | 0.992 | <0.001 | 0.991 | |
| Creatinine | 0.9(0.735,1.22) | 1.16(0.82,2.42) | 1.06 | <0.001 | ||
| Gender | Male | 265(91.9) | 26(8.9) | - | 0.261 | |
| Female | 170(93.9) | 11(6.1) | ||||
| Hyponatremia | 58(82.9) | 12(17.1) | 3.11 | 0.004 | ||
| Pneumonia | 46(65.7) | 24(34.3) | 15.57 | <0.001 | 6.46 | |
| CVA | 18(94.7) | 1(5.3) | _ | 1 | ||
| Sepsis | 12(38.7) | 19(61.3) | 37.12 | <0.001 | 12.42 | |
| HTN | 168(90.3) | 18(9.7) | 0.24 | |||
| CAD | 62(86.1) | 10(13.9) | 2.228 | 0.038 | 3.35 | |
| DM | 152(87.9) | 21(12.1) | 2.44 | 0.008 | ||
| Hypothyroidism | 55(94.8) | 3(5.2) | 0.42 | |||
| Dyslipidemia | 83(96.5) | 3(3.5) | 0.15 | |||
| COPD | 16(84.2) | 3(15.8) | 0.188 | |||
| Bronchial asthma | 20(83.3) | 4(16.7) | 0.207 | |||
| UTI | 32(84.2) | 6(15.2) | 0.058 | |||
| Malignancy | 18(78.3) | 5(21.7) | 3.62 | 0.011 | ||
| CKD | 27(79.4) | 7(20.6) | 3.52 | 0.044 | ||
| CLD | 12(60) | 8(40) | 9.7 | <0.001 | 5.63 | |
Table 1: Results of univariate analysis of risk factors and complications with Outcome.
Note: Quantitative variable –Age was expressed in mean ±SD, all other variables in median (Q1, Q3). Qualitative variables were expressed in number (percentage).
Co-morbidities and outcome
Among the co-morbidities, DM in 63.3% (299) HTN in (60.2%) 284.CAD in (15.2%) 72 Hypothyroidism in 12.3% (58). DLP in (18.2%) COPD in 4%. Bronchial Asthma in 5.1% (24). CKD in 7.2% (34).CLD in 4.2%, Malignancy in 4.9%, CVA in 4.1% of the population
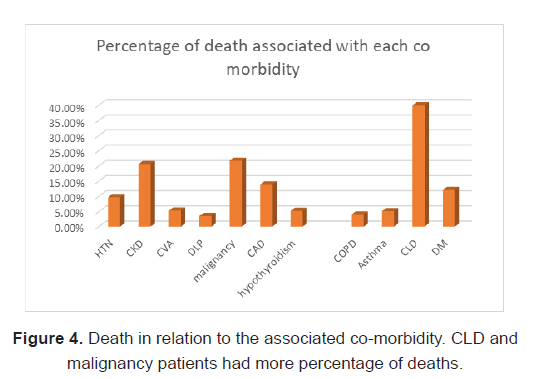
Mortality was seen in 40% in CLD (OR of 5.63), 21.7% in Malignancy (OR of 1.06), 12.1% in Diabetes mellitus (OR of 2.44 in univariate analysis), CAD 13.9% (OR of 2.228 in univariate analysis and 3.35 in multivariate analysis), HTN in 9.7%, 5.2% in hypothyroidism, 3.5% in dyslipidemia, 16.7% in Bronchial Asthma, 15.8% in COPD, 20.6% in CKD, among the 37 patients who died of the disease (Table 2).
| Variables | Sepsis | ||||||
|---|---|---|---|---|---|---|---|
| Present | Absent | Univariate OR | p value | Multivariate OR | |||
| Age | 61.55 ± 13.73 | 54.04 ± 17.9 | 1.02 | 0.008 | 1.03 | ||
| CRP | 52.8(27.4,134.04) | 9.09(2.74,33.55) | 1.009 | <0.001 | |||
| D-dimer | 2.27(1.03,7.16) | 0.53(0.32,1.19) | <0.001 | ||||
| Ferritin | 774.5 (274.3,10250.5) | 205.7(87.37,464.017) | 1 | <0.001 | 1 | ||
| WBC count | 8.1 (5.15,11.75) | 6.69(5.18,8.67) | 1 | 0.075 | - | ||
| NLR ratio | 9.29 (3.46,16.22) | 3.02 (1.78,5.3) | 1.01 | <0.001 | - | ||
| Neutrophil count | 83(66,88) | 67(55.85,76.95) | 1.05 | <0.001 | |||
| Platelet | 173(131,206) | 228 (179,284) | 0.991 | <0.001 | 0.991 | ||
| Creatinine | 1.06(0.81,2.13) | 0.91(0.74,1.23) | 1.096 | 0.048 | |||
| Outcome | Alive | 12(38.7%) | 422(95.9) | <0.001 | |||
| Death | 19 (61.3%) | 18(4.1%) | 37.12 | ||||
Table 2: Association of co- morbidities with sepsis and outcome.
Note: Quantitative variable–Age was expressed in mean ± SD, all other variables in median (Q1, Q3). Qualitative variables were expressed in number (percentage).
Association of co-morbidities with Pneumonia, sepsis and outcome
The D- dimer, ferritin, WBC count, NLR ratio, platelet count and high creatinine 1.16(0.82, 2.42) were statistically different between the alive and the dead. Among the complications, hyponatremia were seen in (17%, ORR-3.11), Pneumonia in (70(14.8%)) Sepsis in (6.6% (31)) and UTI in ((8.1%) 38. The mortality was 34.3%(OR 15.57) in COVID pneumonia, and 61.3% in sepsis (OR 37.12 in univariate and 12.42 in multivariate analysis), 15.2% in UTI (Table 3).
| Variables | PNEUMONIA | |||||
|---|---|---|---|---|---|---|
| Present | Absent | Univariate OR | p value | Multivariate OR | ||
| Age | 58.96 ± 12.34 | 54.04 ± 17.9 | 1.017 | 0.005 | ||
| CRP | 48.48(17.96,103.9) | 8.14(2.54,28.94) | 1.009 | <0.001 | 1.005 | |
| D-dimer | 1.13(0.54,3.17) | 0.51(0.32,1.13) | 1.081 | <0.001 | 1.009 | |
| Ferritin | 619.35(247.45,116..75) | 184.45(84.33,423.02) | 1.001 | <0.001 | 1 | |
| WBC count | 7.42(5.18,10.93) | 6.74(5.17,8.38) | 1 | 0.091 | - | |
| NLR ratio | 5.48(2.74,9.92) | 2.93(1.76,5.15) | 1.009 | <0.001 | - | |
| Neutrophil count | 77.1(65.12,86.32) | 65.05(55.67,76.32) | 1.04 | <0.001 | ||
| Platelet | 203.5(159,266) | 227(177.7,281.2) | 1 | 0.133 | ||
| Creatinine | 1.09(0.79,1.77) | 0.9(0.73,1.22) | 1.06 | 0.015 | ||
| Outcome | Alive | 46(65.7%) | 388(96.8) | <0.001 | ||
| Death | 24 (34.3%) | 13(3.2%) | 15.57 | <15 class="75"></15> | ||
Table 3: Association of co-morbidities with Pneumonia and outcome.
Quantitative variable –All variables in median (Q1, Q3). Qualitative variables were expressed in number (percentage).
Among the 70 COVID patients with pneumonia and 31 patients with sepsis, Age>60 yrs (OR-1.017) CRP (OR-1.009), D-dimer (OR-1.081), ferritin (OR-1.001), neutrophil count (OR-1.04) NLR ratio (OR-1.009) and serum creatinine (OR- 1.06) showed statistical difference between patients who died and who recovered. The Odd’s ratio for CRP (OR-1.005), D-dimer (OR-1.009) and ferritin (OR-1) in multivariate analysis showed significance in pneumonia. High Serum ferritin (OR- 1), low platelet count (OR 0.991) and elderly (OR-1.002) were independent predictors of mortality in sepsis related to COVID.
Discussion
472 in patient cases of COVID admitted in Amrita hospital from 2020 to 2022 were taken into the study. Amrita hospital is a tertiary care hospital located in kerala in South India. Ethics committee approval was obtained on 16/2/2021, No. ECASMAIMS- 2021-105.
The population included mixture of mild, moderate and severe cases. Mean duration of hospital stay was 10.71 during the first and second wave where as it was 8.7 days during the third wave. Mean hospital stay was 13 days for those who died and was 10 days for those who were alive. The difference was not statistically significant. This may be due to the fact that, patients were discharged mandatorily only after COVID rapid antigen negativity during 2020 and 2021 which usually happened by the 10th day after admission and isolation.
Gayatri et al., studied COVID-19 patients between June and August 2020. The response variable was the time from admission to discharge of patients [3]. 730 COVID-19 patients were included, of which 675 (92.5%) recovered and 55 (7.5%) the patient died or was discharged against medical advice. The median length of hospital stay of COVID-19 patients who were hospitalized was found to be 7 days by the Kaplan Meier curve. The covariates that prolonged the length of hospital stay were found to be hypoxia, neutrophil-lymphocyte ratio, D-dimer, lactate dehydrogenase and ferritin. Mortality in our group was 7.8% (Figure 3). In a secondary analysis of an RCT conducted by Jose Mammen et al., where moderately severe COVID patients were studied from 39 public and private hospitals across India from 22 Jan il to 14 July 2020. The cohort of 451 participants with known outcome at 28 days was analysed. The mortality was 14.4%[4]. The demographics, co morbidities and complications were assessed. DM was the most common co- morbidity present in the COVID population studied which was 63.3% followed by systemic hypertension in 60.2%, dyslipidemia in 18.2%, CAD 15.2% (72), Hypothyroidism in 12.3% (58), COPD in 4% (19), Bronchial asthma in 5.1% (24), Chronic kidney disease in 7.2% (34), Malignancy in 4.9% CLD in 4.2% (20), CVA in 4.1%of the population studied.
Considering the association of co-morbidities and death in COVID, 40% of those with CLD expired. (OR of 5.63) and was statistically significant. 13.9% of patients with CAD died, (OR of 2.228 in univariate analysis and 3.35 in multivariate analysis), 21.7% of the population with Malignancy died. (OR of 1.06), 20.6% of patients with CKD, 16.7% of patients with Asthma, 15.8% of COPD, 5.2% of hypothyroidism, 3.5% with DLP, 5.3% with CVA expired. SO mortality was highest among CLD patients in our study (Figure 4).
In a single-centre, retrospective cohort study hospitalized patients with COVID virus infection at Gulu Regional Hospital in Uganda, socio-demographic characteristics, co-morbidities, duration of hospital stay and factors associated with mortality were determined[5]. Co-morbidities included DM, CAD, HIV, COPD and Cancers. Mortality was highest in diabetics (34.4%), cardiovascular diseases (37.5%), hypertensives (31.3%).
Mean age of the patient died was 64.6 ± 1.59 years and the alive patients, it was 53.94 ± 17.28 which was statistically significant. Elderly were prone for COVID associated mortality
In a systematic review of meta-analysis from 120 studies conducted by Biswas et al, it was found that Patients with age ≥ 50 years were associated with 15.4-folds significantly increased risk of mortality compared to patients with age <50 years (RR 15.44: 95% CI 13.02-18.31; p<0.00001) [6].
Of the total population studied, 61.7% (291) were males and 38.3% (181) were females. Among the 37 patients who died, 8.9% (26) of Males and 6.1% (11) of ladies died.
Biswas, et al., meta-analysis review also showed that COVID-19, male patients were associated with significantly increased risk of mortality compared to females (RR 1.86: 95% Confidence Interval (CI) 1.67-2.07; p<0.00001).
In this single-centered, retrospective, observational study, by Wuhan Jin Yin-tan hospital (Wuhan, China), 52 critically ill adult patients with SARS-CoV-2 pneumonia who were admitted to the Intensive Care Unit (ICU) of between late December, 2019, and Jan 26, 2020 were enrolled. Demographic data, symptoms, laboratory values, comorbidities, treatments, and clinical outcomes were all collected. Data were compared between survivors and non-survivors. The primary outcome was 28-day mortality, as of Feb 9, 2020 [7].
Of 710 patients with SARS-CoV-2 pneumonia, 52 critically ill adult patients were included. The mean age of the 52 patients was 59.7 (SD 13.3) years, 35 (67%) were men, 21 (40%) had chronic illness, 51 (98%) had fever. 32 (61.5%) patients had died at 28 days, and the median duration from admission to the Intensive Care Unit (ICU) to death was 7 (IQR 3–11) days for non-survivors. Compared with survivors, non-survivors were older (64.6 years (11.2) vs. 51.9 years (12.9)), more likely to develop ARDS (26 (81%) patients vs. 9 (45%) patients)
Though there was a considerable difference in the median CRP, D-dimer, WBC count, NLR ratio, Platelet count, creatinine in the patients who were alive and who survived the disease, only D-dimer, Total WBC count, NLR ratio and platelet count were statistically significant while considering mortality.
In a systematic meta-analysis by Giovanni Ponti, total of 83 studies were included. Random effect model results showed that patients with severe COVID-19 group had higher levels of C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), Procalcitonin (PCT), Interleukin-6 (IL-6), Interleukin-10 (IL-10), Interleukin-2R (IL-2R), Serum Amyloid A (SAA) and Neutrophil-To-Lymphocyte Ratio (NLR) compared to those in the non-severe group [8]. Similarly, the fixed-effect model demonstrated significant higher ferritin level in the severe group when compared with the non-severe group. The random effect model results demonstrated that the non-survivor group had significantly higher levels of CRP, PCT, IL-6, ferritin, and NLR when compared with the patients who survived.This stresses the importance of immune system activation and increase of inflammatory markers in severe COVID.
In 31 COVID patients with sepsis, Median CRP (52 vs 9), D-dimer (2.27 vs. 0.53), ferritin (774 vs. 205), NLR ratio (9.29 vs. 3) neutrophil percentage (83 vs. 67), low platelet count (173 vs. 228) were all statistically different in those who died, i.e 19 (61.3%) from those who were alive 38.7% (12). 61.3% of patients with sepsis died which had an odd’s ratio of 37.12 in univariate analysis contributing significantly to death [9].
In a study by Mutair et al., it was found that 86 patients with COVID-19 admitted in ICU, 50 patients died, 23 recovered, and 13 were still admitted, with a mortality rate of 58.1% [10]. Septic shock (OR (95% CI): 58.1 (5.97–7812.8), p<0.001) and Acute Kidney Injury (AKI) (OR (95% CI): 7.279 (1.191– 65.43), p=0.032) had a significant impact on mortality. Cox proportional-hazards regression analysis revealed that septic shock (HR (95% CI): 9.502 (2.958–30.524), p<0.001) and neutrophil count (HR (95% CI): 1.053 (1.023–1.085), p<0.001) were significant predictors for mortality.
Conclusion
Males and elderly had a higher mortality rate. Serum Ferritin, D-dimer and NLR ratio were higher in patients who died. Though Diabetes mellitus was the most common co morbidity, patients with Cirrhosis and malignancy showed significant predictors of mortality. Pneumonia and septicaemia proved to be the most fatal complications in patients infected with COVID- 19. There was no significant difference as far as hospital stay was concerned.
Acknowledgements
I acknowledge my sincere thanks to all the house surgeons who helped me with the data collection.
References
- UdwadiaZF, Tripathi AR, Nanda VJ, Joshi SR. Prognostic factors for adverse outcomes in COVID-19 infection. J Assoc Physicians India. 2020;68:62-66.
[Google Scholar], [Indexing]
- Garcia PDW, Fumeaux T, Guerci P, Heuberger DM, Montomoli J, et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EclinicalMedicine. 2020;25:100449.
[Crossref] [Google Scholar], [Indexing]
- Thiruvengadam G, Lakshmi M, Ramanujam R. A study of factors affecting the length of hospital stay of COVID-19 patients by cox-proportional hazard model in a south indian tertiary care hospital. J Prim Care Community Health. 2021; 12: 21501327211000231.
[Crossref] [Google Scholar], [Indexing]
- Mammen JJ, Kumar S, Thomas L, Kumar G, Zachariah A. Factors associated with mortality among moderate and severe patients with COVID-19 in India: A secondary analysis of a randomised controlled trial. BMJ Open. 2021;11:e050571.
[Crossref] [Google Scholar], [Indexing]
- Baguma S, Okot C, Alema NO, Apiyo P, Layet P, et al. Factors associated with mortality among the COVID-19 patients treated at gulu regional referral hospital: A retrospective study. Front Public Health. 2022; 10:841906.
[Crossref] [Google Scholar], [Indexing]
- Biswas M, Rahaman S, Biswas TK, Haque Z, Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: A systematic review and meta-analysis. Intervirology. 2020;9;1-12.
[Crossref] [Google Scholar], [Indexing]
- Yang X, Yu Y, Xu J, Shu H, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475-481.
[Crossref] [Google Scholar], [Indexing]
- Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389-399.
[Crossref] [Google Scholar], [Indexing]
- Garg M, Maralakunte M, Garg S, Dhooria S, Sehgal I, et al. The Conundrum of 'Long-COVID-19': A narrative review. Int J Gen Med. 2021;14:2491-2506.
[Crossref] [Google Scholar], [Indexing]
- Al Mutair A, Al Mutairi A, Zaidi ARZ, Salih S, Alhumaid S, et al. Clinical Predictors of COVID-19 Mortality among Patients in Intensive Care Units: A Retrospective Study. Int J Gen Med. 2021;14:3719-3728.
[Crossref] [Google Scholar], [Indexing]

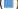 ) males;(
) males;( ) females
) females



 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.