C‑reactive Protein and Disease Outcome in Nigerian Sickle Cell Disease Patients
- *Corresponding Author:
- Dr. Okocha EC
Department of Haematology, Nnamdi Azikiwe University Teaching Hospital, Nnewi PMB 5025, Anambra State, Nigeria.
E-mail: onyichideokocha@yahoo.com
Abstract
Background: Evidence suggests that sickle cell disease (SCD) is associated with a chronic inflammatory state. C‑reactive protein (CRP) is known to modulate inflammation. Its role in the chronic inflammation of SCD may make it valuable as a therapeutic target. Aim: The aim was to determine CRP levels in SCD subjects in asymptomatic steady state (ASS) and crisis and correlate these with severity scores in the ASS. Subjects and Methods: We measured the level of CRP in 30 hemoglobin SS (HbSS) individuals in ASS and seven in crisis. As controls, we measured CRP in 50 individuals each who were hemoglobin AS and hemoglobin AA respectively, using enzyme linked immunoabsorbent assay based commercially available kits from East Wing Diagnostic Limited Full blood count (white blood cell [WBC]) was done for the ASS HbSS individuals using a cell counter and their disease severity calculated by an objective scoring method. Results: Our results showed that ASS HbSS individuals had significantly higher CRP levels compared with the controls. The HbSS individuals in crisis also had a significantly higher level of CRP compared to the ASS HbSS individuals. Disease severity and WBC were found to be negatively correlated with CRP levels (P = 0.17; and 0.73, respectively). Conclusion: Our results suggest that increased levels of CRP in ASS HbSS individuals may play a protective role in SCD leading to better disease outcome, and may have value as a therapeutic target.
Keywords
Chronic inflammation, C-reactive protein, Disease severity, Sickle cell disease, Therapeutic target
Introduction
The symptoms and complications of sickle cell disease (SCD) arise mainly from the crises (clinical and subclinical). Activation and damage of endothelial cells with activation of adhesion molecules lead to inflammation, release of C-reactive protein (CRP) and other inflammatory mediators and subsequent enhancement of ischemia.[1] The above and other lines of evidence suggest that SCD is associated with a chronic inflammatory state,[2] in which inflammation, oxidative stress and tissue oxidative damage occur, leading to various degrees of disease severity and end-organ dysfunction. Exploring the role of CRP in this chronic inflammatory state is very important as we search for therapeutic targets in this disease. We therefore measured serum levels of CRP in asymptomatic steady state (ASS) and crises situations in Nigerian SCD patients and related these to objective severity scores in the ASS.
Subjects and Methods
Patient selection
One hundred and thirty subjects randomly selected were recruited for this study between March 2012 and October 2012, including 30 homozygous ASS SCD patients hemoglobin SS (HbSS) selected randomly from our sickle cell clinic. Steady state condition was defined as no manifest crisis for at least 4 weeks after the last episode, 3 or more months after the last blood transfusion and no febrile episode for at least 2 weeks. Seven out of these were also studied during the vaso-occlusive crisis (VOC). Fifty subjects with sickle cell trait hemoglobin AS (HbAS) and 50 subjects homozygous for hemoglobin AA (HbAA) who were apparently healthy were also randomly selected to serve as controls. Written informed consent was obtained from the subjects or their parents/caregiver. Ethical approval for the study was obtained from the Hospital Ethics Committee. Demographic and phenotypic data such as age, sex, and last episode of febrile illness and complications of SCD (if any) were also recorded. Complications were such events as priapism, stroke, and avascular necrosis of the femoral head or bone, ankle ulcers or any other condition complicating the disease. Most of the HbSS patients were on routine drugs such as folic acid supplements, antimalarial prophylaxis, low dose soluble aspirin, and omega-3 fatty acids.
Disease severity in hemoglobin SS patients
Disease severity was determined by calculating an objective score using a modification of the method described by Hedo et al.[3] Points were assigned for the following characteristics: Degree of anemia, number of complications and number of transfusions. Scores of ≤2 were considered mild disease. Scores of 2 ≥ 5 were deemed as moderate disease, while scores >5 were considered severe disease.
Sample collection and laboratory analysis
A sample of 5 ml of blood was collected from each participant, from which 3 ml was dispensed into ethylene diamine tetra acetic acid for determination of full blood count (FBC) and 2 ml into plain tubes for determination of CRP. FBC was done using a 17 parameter, 3-part WBC Deferential, Automated Hematology analyser (KX-21N, Sysmex corporation, Chuo-ku, Kobe, Japan). The parameters determined included packed cell volume, hemoglobin, platelet count, total white blood cell count, and differential white blood cell (WBC) count.
Hemoglobin phenotype was determined using cellulose acetate paper electrophoresis (Kohn 1960). The serum level of CRP was assayed using commercially available CRP kits (East Wing Diagnostic Limited). This assay was based on enzyme linked immunoabsorbent assay.
Statistical analysis
Data obtained were analyzed using Statistical Package for Social Sciences software package version 20 (SPSS Inc., IL, Chicago, USA). Values obtained were tabulated by age group and sex. The Chi-square or Mann–Whitney U-test - (depending on whether the data were skewed) - was used to compare frequencies and generate P values. Pearson’s and Spearman’s correlation tests were used to determine the correlation between variables. P < 0.05 were considered as significant.
Results
Thirty steady state HbSS were studied. Nine of them were nonpregnant females, while 21 were males. Their ages were between 5 and 38 years, mean (SD) of 19 (9.4). The control group consisted of 100 apparently healthy subjects. Fifty of them were hemoglobin phenotype AS while the rest had hemoglobin phenotype AA. They were of similar age, sex, and socioeconomic class distribution as the HbSS subjects. Of the 30 HbSS subjects studied, three met the criteria for mild disease; 15 had moderately severe disease, while 12 had severe disease [Table 1].
| Disease severity | Hemogram parameters and CRP | |||||
|---|---|---|---|---|---|---|
| CRP Median | WBC×1000/ul Mean (SD) | Hb (g/dl) Median (SD) | MCHC (g/dl) Mean (SD) | MCV (fl) Mean (SD) | MCH (pg) Mean (SD) | |
| Mild | 8.00 | 13.77 (3.92) | 11.00 | 32.90 (1.21) | 80.00 (1.95) | 26.80 (0.50) |
| Moderate | 4.00 | 14.27 (9.18) | 9.30 | 32.06 (1.07) | 80.54 (8.11) | 26.29 (2.63) |
| Severe | 4.00 | 12.00 (8.60) | 8.05 | 32.26 (1.56) | 84.46 (9.62) | 28.73 (4.61) |
| P value | 0.38 | 0.69 | 1.00 | 0.29 | 0.39 | 0.26 |
CRP: C‑reactive protein, MCV: Mean corpuscular volume, MCH: Mean corpuscular hemoglobin, MCHC: Mean corpuscular hemoglobin concentration, WBC: White blood cell, HbSS: Hemoglobin SS, SD: Standard deviation, ASS: Asymptomatic steady state
Table 1: Disease severity versus median CRP and Hb with mean MCH, MCV and MCHC in ASS HbSS group
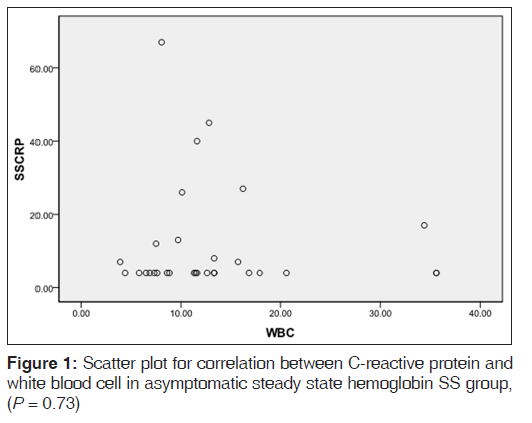
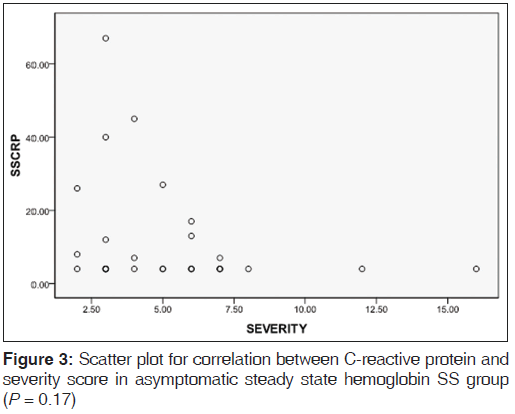
Table 2 shows the mean levels of CRP in the various hemoglobin phenotype groups. CRP level was significantly raised in the ASS HbSS group when compared to the control group (P = 0.001). CRP levels in the ASS HbSS group showed a negative correlation with disease severity scores with P value being close to significance (P = 0.17) [Figure 2] WBC also showed a negative correlation with CRP levels in the same group of subjects (P = 0.73), [Figure 3].
| Study groups | Mean of CRP values | P |
|---|---|---|
| AA/AS | 1.97/3.40 | <0.001 |
| AA/SS | 1.97/11.50 | <0.001 |
| AS/SS | 3.40/11.50 | <0.001 |
| SS in crisis/SS not in crisis | 22.71/7.86 | 0.03 |
| CRP: C‑reactive protein |
Table 2: Comparison of the mean values of CRP in the various genotype groups
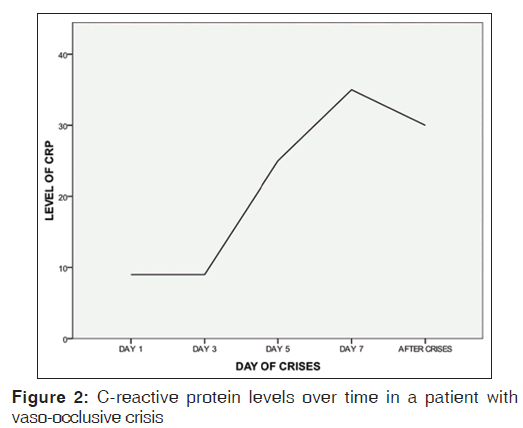
Table 1 shows CRP levels and FBC values for ASS HbSS individuals. Figure 1 is a line graph of changes in CRP values over time in a HbSS patient in VOC.
Discussion
We have shown that ASS HbSS individuals in Nnewi have significantly highekr CRP levels compared with HbAA or AS individuals. In crisis, HbSS individuals had a further significant rise of CRP levels when compared to ASS HbSS individuals. Interestingly, in ASS HbSS individuals, disease severity and WBC correlated negatively with CRP levels, although this did not reach statistically significant levels (P = 0.17 and 0.73, respectively).
Our finding that CRP is significantly increased in crisis compared with the stable state in HbSS individuals and in HbSS compared with HbAS and HbAA individuals is corroborated by other workers.[3-5] CRP production is part of a nonspecific acute phase response to inflammation and tissue necrosis, we are therefore not surprised that its level is significantly higher in ASS HbSS individuals; as SCD has been associated with a chronic inflammatory state.[2] The hypothesis here is that repeated obstruction of blood vessels and eventual reperfusion of necrosed tissue lead to the formation of oxygen radicals, which damage the tissues and set up a low level state of ongoing inflammation.[2] It is tempting to hypothesize that the genetic propensity a HbSS individual possesses to produce CRP in the ASS, may be able, in part, to predict disease outcome.[6]
The VOC crisis state is a phase where there is an acute increase of vaso occlusion and tissue necrosis. Hence, we find a significant rise in CRP levels above the ASS of HbSS individuals. In our data set, we found that this rise followed over time, tended to get to a peak as the crisis progressed and fall rapidly as it abates. This led Stuart et al., 1994, to propose that change in CRP levels may have potential value in monitoring the progress or resolution of a crisis.[4] Our data are in complete agreement with this proposal [Figure 2].
Our finding that the disease severity scores correlated negatively with CRP levels among ASS HbSS individuals [Figure 3], was at variance with the findings of Hedo et al.[3] and Krishnan et al.[7] On the surface, it may seem to make sense that there should be a positive correlation between increased levels of CRP and disease severity; since it would seem that higher CRP levels would be a marker for higher levels of inflammation and therefore increased disease severity. However, a closer look at this hypothesis reveals flaws, especially in the light of the question; is CRP just a marker or does it play a causal role in the pathogenesis of inflammation associated with SCD?
Many lines of evidence suggest that CRP is a marker and not a maker in the pathogenesis of inflammation involved in many disorders.[8-10] In addition to this, elevated levels of CRP may well provide an important part of nonspecific host defense mechanism especially in the early stages of inflammation.[11] Although the biological role of CRP and its relation to the inflammatory process is not fully certain, evidence has been presented that it stimulates leukocyte migration,[12] reacts with bacterial surfaces to facilitate phagocytosis,[13] signals an enhanced immune response,[14] induces lymphocyte blast transformation,[15] or enhances lymphocyte cytotoxicity,[15] and has a protective effect on endothelium derived nitric oxide (EDNO) bioavailability[8] - lack of EDNO due to excessive hemolysis is a major pathogenic pathway in the development of pulmonary hypertension as a complication of SCD.[16] The above and other evidence suggest that the biologic role of CRP in relation to the inflammatory process may be protective in ASS HbSS individuals. Hence, our finding that it correlates negatively with disease severity.
Zacho et al., 2008, showed that individuals are assigned into two groups genetically with higher or lower levels of CRP depending on the genetic variants they receive from their parents.[17] We hypothesize that ASS HbSS individuals assigned to higher CRP levels may have a better disease outcome because higher CRP levels enables their immune system to respond better to challenge. To the best of our knowledge, we are the first to present evidence for this hypothesis. Clearly, our hypothesis is limited to only ASS HbSS patients and may not apply in acute situations like crisis where CRP response to the acute condition may blunt the differences between the two groups of individuals.
Our finding is in agreement with the report of Walter et al.,[18] who noted a protective role of inflammation in SCD. They reported that SCD patients had a higher degree of the inflammatory response, evidenced by higher levels of inflammatory mediators and lower levels of markers of oxidative tissue damage and levels of nontransferrin bound iron, compared to patients with thalassemia. They thus hypothesized that the protective role of the exaggerated inflammatory response in SCD stems from its increased iron cycling and sequestration into reticuloendothelial cells and thus low free serum iron, which is toxic to tissues. This study also found significantly higher levels of CRP in SCD patients compared to thalassemia patients or controls. The WBC, has been well-documented as an index of severe outcome in ASS HbSS individuals,[2] we found a negative correlation between WBC and CRP levels in our study population, even though the P value did not reach statistical significance (P = 0.73), it appears to further show that the relationship between CRP and disease severity in ASS HbSS individuals is a negative one. Microcytosis observed in some of our subjects is more a marker for iron deficiency than for thalassemia syndromes;[19] hence, this does not introduce a confounding factor in the severity assessment of our SCD patients.
The work of Krishnan et al.,[7] was retrospective, and they correlated CRP with VOC events. A VOC event alone is inadequate to judge disease severity. We also modified the method Hedo et al.,[3] used to compute disease severity to make it more robust. Our work was however limited by the fact that some parameters we used depended on the ability of our patients to recall and that just a single measurement of CRP was made in the SCD population.
Conclusion
Our data seem to suggest that the biologic role of CRP in relation to the inflammatory process in ASS HbSS patients is a protective one. As yet, we do not know how well CRP will turn out as a therapeutic target, but clearly, more work needs to be done to confirm our findings with a larger population of HbSS individuals with serial longitudinal measurements of CRP and other markers of inflammation. More HbSS individuals in crisis also need to be observed longitudinally until their CRP levels come back to baseline.
Acknowledgments
This work was supported by the individual contributions of the authors. We wish to acknowledge our patients, other subjects and the data collection team who under very challenging conditions were very cooperative.
References
- Manwani D, Frenette PS. Vaso-occlusion in sickle cell disease: Pathophysiology and novel targeted therapies. Blood 2013;122:3892-8.
- Bandeira IC, Rocha LB, Barbosa MC, Elias DB, Querioz JA, Freitas MV, et al. Chronic inflammatory state in sickle cell anemia patients is associated with HBB(*)S haplotype. Cytokine 2014;65:217-21.
- Hedo CC, Aken’ova YA, Okpala IE, Durojaiye AO, Salimonu LS. Acute phase reactants and severity of homozygous sickle cell disease. J Intern Med 1993;233:467-70.
- Stuart J, Stone PC, Akinola NO, Gallimore JR, Pepys MB. Monitoring the acute phase response to vaso-occlusive crisis in sickle cell disease. J Clin Pathol 1994;47:166-9.
- Pradhana RK, Mishraa R, Naga C. Diurnal variations of C-reactive protein in trait and sickle cell disease patients. Biol Rhythm Res 2013;44:277-85.
- Milton JN, Gordeuk VR, Taylor JG 6th, Gladwin MT, Steinberg MH, Sebastiani P. Prediction of fetal hemoglobin in sickle cell anemia using an ensemble of genetic risk prediction models. Circ Cardiovasc Genet 2014;7:110-5.
- Krishnan S, Setty Y, Betal SG, Vijender V, Rao K, Dampier C, et al. Increased levels of the inflammatory biomarker C-reactive protein at baseline are associated with childhood sickle cell vasocclusive crises. Br J Haematol 2010;148:797-804.
- Holmes MV, Jiang B, McNeill K, Wong M, Oakley SP, Kirkham B, et al. Paradoxical association of C-reactive protein with endothelial function in rheumatoid arthritis. PLoS One 2010;5:e10242.
- Schunkert H, Samani NJ. Elevated C-reactive protein in atherosclerosis – chicken or egg? N Engl J Med 2008;359:1953-5.
- Koike T, Kitajima S, Yu Y, Nishijima K, Zhang J, Ozaki Y, et al. Human C-reactive protein does not promote atherosclerosis in transgenic rabbits. Circulation 2009;120:2088-94.
- Ceciliani F, Giordano A, Spagnolo V. The systemic reaction during inflammation: The acute-phase proteins. Protein Pept Lett 2002;9:211-23.
- El Kebir D, Zhang Y, Potempa LA, Wu Y, Fournier A, Filep JG. C-reactive protein-derived peptide 201-206 inhibits neutrophil adhesion to endothelial cells and platelets through CD32. J Leukoc Biol 2011;90:1167-75.
- Lu J, Marjon KD, Marnell LL, Wang R, Mold C, Du Clos TW, et al. Recognition and functional activation of the human IgA receptor (FcalphaRI) by C-reactive protein. Proc Natl Acad Sci U S A 2011;108:4974-9.
- Coventry BJ, Ashdown ML, Quinn MA, Markovic SN, Yatomi-Clarke SL, Robinson AP. CRP identifies homeostatic immune oscillations in cancer patients: A potential treatment targeting tool? J Transl Med 2009;7:102.
- Vetter ML, Gewurz H, Hansen B, James K, Baum LL. Effects of C-reactive protein on human lymphocyte responsiveness. J Immunol 1983;130:2121-6.
- Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, et al. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA 2005;294:81-90.
- Zacho J, Tybjaerg-Hansen A, Jensen JS, Grande P, Sillesen H, Nordestgaard BG. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med 2008;359:1897-908.
- Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, Madden J, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol 2006;135:254-63.
- Akinbami AA, Dosunmu AO, Adediran AA, Oshinaike OO, Osunkalu VO, Ajibola SO, et al. Serum ferritin levels in adults with sickle cell disease in Lagos, Nigeria. J Blood Med 2013;4:59-63.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.