Cytokeratin 19 Expression Patterns of Dentigerous Cysts and Odontogenic Keratocysts
- *Corresponding Author:
- Dr. Kavitha P Kamath
Department of Oral Pathology, People’s Dental Academy, Bhanpur, Bhopal ‑ 462 037, Madhya Pradesh, India.
E‑mail: kavithapkamath@yahoo.co.in
Abstract
Background: Although numerous investigators have studied the pattern of keratin expression in different odontogenic cysts, the results have been variable. Aim: The present study was conducted to determine the pattern of expression of cytokeratin 19 (CK 19) in the epithelial lining of odontogenic keratocysts and dentigerous cysts. Materials and Methods: The epithelial layers showing expression of the epithelial marker CK 19 was determined by immunohistochemical methods in 15 tissue specimens each of histopathologically confirmed cases of dentigerous cysts and odontogenic keratocysts. Statistical analysis was done to compare the CK 19 expression between dentigerous cyst and odontogenic keratocyst using the Chi‑square test. P < 0.05 was considered to be statistically significant. Results: All specimens of dentigerous cysts were positive for CK 19 with 20% (3/15) of the specimens showing expression only in a single layer of the epithelium, 40% (6/15) of the specimens showing expression in more than one layer but not the entire thickness of the epithelium, and the remaining 40% (6/15) showing expression throughout the entire thickness of the epithelium. In the case of odontogenic keratocysts, 40% (6/15) of the specimens were negative for CK 19, 40% (6/15) of the specimens showed expression only in a single layer of the epithelium, and 20% (3/15) of the specimens showed expression in more than one layer, but not the entire thickness of the epithelium. The observed differences in CK 19 expression by the two lesions were statistically significant (P < 0.01). Conclusion: The differences in CK 19 expression by these cysts may be utilized as a diagnostic tool in differentiating between these two lesions.
Keywords
Dentigerous cyst, Immunohistochemistry, Keratins, Odontogenic cyst
Introduction
Different odontogenic cysts have variable clinical and biological behaviors. The odontogenic keratocyst (also termed as keratocystic odontogenic tumor), which occurs mainly in the mandibular posterior region, is an aggressive cystic lesion which has received special attention in the dental literature due to its high recurrence rate which ranges from 3% to 60%.[1] In contrast, the dentigerous cyst, which usually encloses the crown of an unerupted tooth, usually does not recur after adequate treatment. On account of their different biological behavior which necessitates different treatment approaches, it is very essential that a clear differentiation be made between the two lesions at the time of diagnosis. It has been proposed that specific properties of the cyst epithelium or supporting connective tissue, or both may explain the differences in the clinical behavior of the odontogenic keratocyst and other odontogenic cysts. Consequently, numerous attempts have been made to differentiate odontogenic keratocysts from dentigerous cysts by studying the patterns of expression of various molecular components, notably cytokeratins (CKs), by immunohistochemical methods.[2‑6]
Cytokeratins that form the cytoskeleton of the epithelial cells are of several molecular types. The patterns of expression of these different types of CKs vary depending upon the type of epithelial cells and hence, they may be used as potential markers of cell differentiation and malignant transformation. CK 19, a type I (acidic) keratin, is the smallest keratin and is unique in that it lacks the carboxyterminal, non‑α‑helical tail domain, which is typical for all other keratins.[7] Although numerous investigators have studied the pattern of keratin expression in different odontogenic cysts, the results have been variable.[8‑12] Gao et al.[8] studied the patterns of expression of different CK in dentigerous cysts and keratocysts and reported that CK 19 stained with either the full thickness of the epithelial lining or with only the superficial cell in the dentigerous cysts while it stained suprabasal cells and some basal cells in odontogenic keratocysts. They also reported that while CK 4 and CK 13 stained the suprabasal cells of both dentigerous and odontogenic keratocysts, CK 16 reacted only weakly and patchily with suprabasal cells in some dentigerous cysts while it stained strongly with the suprabasal cells of all odontogenic keratocysts. Another study by Hormia et al. reported that antibodies to CK 19 reacted with the basal and parabasal cells and antibodies to CK 13 and 16 reacted with the suprabasal cells in odontogenic kertatocyts while they showed heterogenous positivity in dentigerous cysts.[10] While antibodies to CK 18 did not stain any of the cells in odontogenic keratocysts, dentigerous cysts showed a distinct layer of cells positive to CK 18. Stoll et al. reported that while greater proportion of odontogenic keratocysts in their study showed expression of CK 17 compared to dentigerous cysts, CK 19 expression was not demonstrated in any of the odontogenic keratocysts while it was present in around 50% of the dentigerous cysts.[2] On account of these variations in the results reported by different investigators, the present study was conducted to determine the pattern of expression of CK 19 in the epithelial lining of odontogenic keratocysts and dentigerous cysts.
Materials and Methods
The present study was conducted as a retrospective study utilizing tissue specimens from the archives of the Department of Oral Pathology, Yenepoya Dental College, Mangalore, Karnataka State, India during the period July 2008 to January 2009. Fifteen tissue specimens each of histopathologically confirmed cases of dentigerous cysts, as well as odontogenic keratocysts (nonsyndromic), were utilized for the purpose of the study. No formal power analysis was done in calculating the sample size, and a convenience sampling technique was followed. Tissue specimens that were smaller than 3 mm in size were not considered for the study purpose. The study protocol was approved by the Institutional Ethics Committee of Yenepoya Dental College.
The cytoplasmic expression of the epithelial marker CK 19 in the lining epithelium of dentigerous cysts and odontogenic keratocysts was determined by immunohistochemical staining in which Super Sensitive Multilink‑Horseradish Peroxidase (HRP) Detection Kit/Diaminobenzidine (DAB) (BioGenex Laboratories Inc., San Ramon, California, USA) was used for determining the expression of CK 19 in the tissue specimens. Tissue sections of 3–4 μm thickness were obtained by cutting the tissue blocks over rotary microtome (LEICA RM; Leica Microsystems Inc., Buffalo Grove, Illinois, USA) and mounted on to 3‑aminopropyltriethoxysilane coated slides. The sections were deparaffinized with xylene, rehydrated in grades of alcohol, and washed in distilled water.
Antigen retrieval was performed by treatment with the enzyme pepsin at a temperature of 35–40°C for 12 min. The pepsin solution for the antigen retrieval was prepared according to the recommendation of the manufacturer by dissolving 800 mg of pepsin powder in 40 ml of distilled water and adjusting the pH to 2.5 with 1 N HCl.
After antigen retrieval, the tissue sections were rinsed first in distilled water and then with Tris buffer, followed by treatment with peroxidase block (3% hydrogen peroxide) for 15 min to block endogenous peroxidase activity. The slides were then placed in a humid chamber. After this, the slides were drained and rinsed in two changes of Tris buffer (wash buffer), each of 5 min duration. Nonspecific protein–protein interactions were blocked by treating and incubating the tissue sections with the power block (casein in phosphate buffered saline) for 10 min duration in a humid chamber. After this, the remaining solution was drained from the slides. The sections were then incubated in the prediluted primary antibody (CK 19) at room temperature in the humid chamber for 90–120 min. The primary antibody provided by the manufacturer was mouse monoclonal antibody (class IgG1 kappa, clone RCK108) to CK 19 from ascites and reacts with the 40 kDa protein corresponding to human CK 19. Again, the remaining solution was drained from the slides and rinsed in two changes of Tris buffer (washing buffer) as mentioned earlier. Sections were then treated with a reagent for enhancing the staining for 30 min, followed by rinse in two changes of Tris buffer. Sections were then incubated with multilink secondary antibody solution (biotinylated anti‑immunoglobulins) for 30 min. This was followed by rinsing with wash buffer and treatment with HRP label (HRP‑conjugated streptavidin) for 30 min. Subsequently, the sections were treated with DAB chromogen/substrate 3,3‑DAB chromogen/H2O2/substrate buffer solution (1 ml of DAB buffer mixed with 1 drop of DAB chromogen in the mixing vial and allowed to stand for about 10 min), covered with drops of chromogen DAB buffer solution, and allowed to stand in the humid chamber for 8–10 min. During this period, brown staining was visible with varying intensity on different sections. Then the slides were rinsed first in distilled water followed by running tap water. The sections were then counterstained with Harris hematoxylin solution for 20 s, rinsed in running tap water, and dehydrated and mounted with dibutyl phthalate and xylene. Colon carcinoma tissue sections were used as positive controls while specimens that were treated as above except for the fact that the primary antibody was omitted were used as negative controls.
The 30 stained slides were evaluated under the bright field (LABOMED; Labomed, Inc., Los Angeles, California, USA) at 100‑ and 200‑fold magnification. The extent of cytoplasmic staining was graded as follows:
• ‘+’ ‑ Single layer of the lining epithelium is stained
• ‘++’ ‑ More than one layer of the lining epithelium is stained, but not the entire thickness
• ‘+++’ ‑ Entire thickness of the lining epithelium is stained.
The tissue sections which did not express CK 19 were considered as negative in pattern of staining. Photomicrographs were taken with Olympus SP‑350 camera (Olympus Imaging America Inc., Center Valley, Pennsylvania, United States).
Statistical analysis was performed to compare the CK 19 expression between dentigerous cyst and odontogenic keratocyst using the Chi‑square test. Statistical analyses were performed using software for statistical analysis (SPSS software version 17, IBM Corporation, Armonk, New York, USA). P < 0.05 was considered as statistically significant.
Results
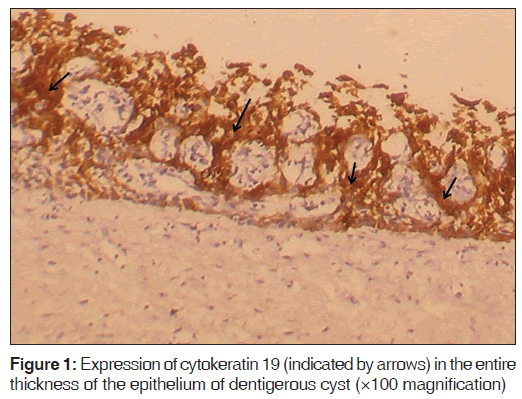
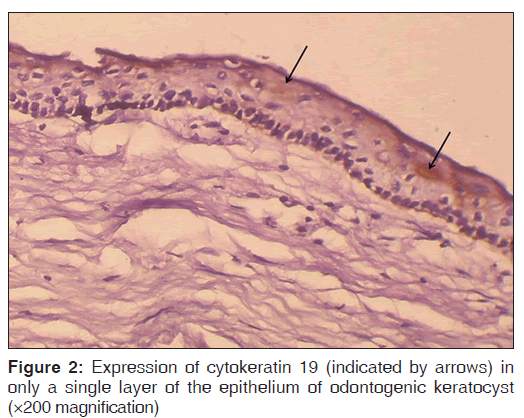
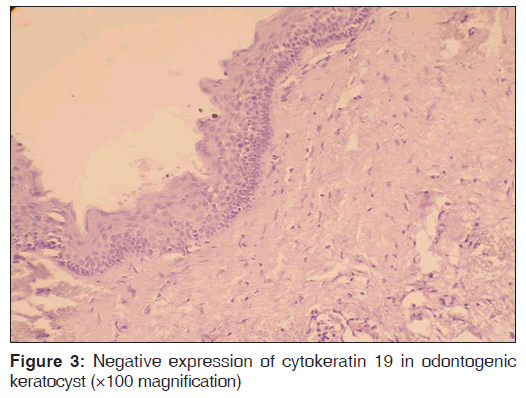
The number of specimens of dentigerous cysts and odontogenic cysts showing different patterns of staining is shown in Table 1. Among the 15 specimens of dentigerous cysts, 20% (3/15) showed ‘+’, 40% (6/15) showed ‘++’, and 40% (6/15) showed ‘+++’ expression [Figure 1] of the CK 19. The specimens that showed ‘+’ and ‘++’ staining showed staining of mainly the superficial and supra basal cells. Regarding the odontogenic keratocysts, among the 15 specimens, 40% (6/15) showed ’+’ [Figure 2], 20% (3/15) showed ‘++’, and 40% (6/15) were negative [Figure 3] for the expression of CK 19. The CK 19 positive specimens showed staining of mainly the superficial layer of epithelial cells. The observed differences in the pattern of vertical extent of CK 19 expression between dentigerous cysts and odontogenic keratocysts were statistically significant (P < 0.01).
| Type of cyst (n=15) | ‘+’ % (n) | ‘++’ % (n) | ‘+++’ % (n) | ‘Negative’ % (n) | Total % (n) |
|---|---|---|---|---|---|
| Dentigerous cyst | 20 (3) | 40 (6) | 40 (6) | 0 | 100 (15) |
| Odontogenic keratocysts | 40 (6) | 20 (3) | 0 | 40 (6) | 100 (15) |
P<0.01. ‘+’: Single layer of lining epithelium is stained, ‘++’: More than one layer of lining epithelium is stained but not the entire thickness, ‘+++’: Entire thickness of lining epithelium is stained
Table 1: Patterns of cytokeratin 19 expression in dentigerous cysts and odontogenic keratocysts
Discussion
On account of the differences in the clinical behavior such as chances of recurrence of the dentigerous cysts and odontogenic keratocysts, it is very important that a clear differentiation may be made between the two entities. Since these lesions arise from odontogenic epithelium and may have a similarity in the radiographic and histological appearance, numerous attempts have been made to differentiate these two lesions by immunohistochemical methods targeting various molecules including CKs.
Several studies have been carried out by different researchers to determine if particular patterns of CKs would serve as accurate diagnostic markers for the odontogenic keratocysts and the dentigerous cysts. The various CKs that have been studied include CK 4, 5, 6, 7, 8, 10, 13, 14, 16, 17, 18, 19, and 20.[2,8,10,11]
In the present study, the pattern of expression of CK 19 in odontogenic keratocysts and dentigerous cysts was studied immunohistochemically, and it was observed that CK 19 expression was more pronounced in dentigerous cysts than in odontogenic keratocysts. CK 19, the smallest known acidic type CK, is expressed in human tissues without association with a basic CK.[7] Usually expressed in the basal cells of nonkeratinizing stratified squamous epithelia,[13] CK 19 expression has been reported to occur in the suprabasal cells of oral stratified squamous epithelium in association with inflammation and epithelial dysplasia.[14,15] CK 19 expression in various pathologic conditions has been studied previously. CK 19 expression has been associated with poor differentiation and aggressive behavior of hepatocellular carcinomas[16] and has been used for differentiating hepatocellular carcinoma from adenocarcinoma.[13] CK 19 expression has also been reported to be higher in malignant neoplasms of the thyroid compared to benign nodules.[17] Detection of soluble fragments of CK 19 in the serum has been used as a marker for monitoring treatment and response to therapy of squamous cell carcinoma[13] and it has been demonstrated that tumor cells in breast cancer patients can release full‑length CK 19 and this is associated with high metastatic properties.[18] Detection of high levels of fecal CK 19 mRNA has also been suggested as a potential marker for colorectal malignancy.[19] CK 19 expression has been reported to be reduced in oral submucous fibrosis compared to healthy mucosa and CK 19 gene is one of the genes with decreased expression in oral submucous fibrosis.[20]
The pattern of expression of CK 19 in odontogenic keratocysts and dentigerous cysts has been previously investigated. Hormia et al., in their study with immunohistochemical staining of odontogenic keratocysts and dentigerous cysts for CK 19 showed that the basal and few suprabasal cells of odontogenic keratocysts showed positive expression whereas, in the case of dentigerous cysts, the antibody to CK 19 showed heterogeneous positivity with the staining pattern varying between different samples, within the same sample and between the cells.[10] Gao et al., in their study, reported that the antibody to CK 19 reacted with either the full thickness of the epithelium or only the superficial cells in the case of dentigerous cysts and with the supra basal cells and some basal cells in the case of odontogenic keratocysts.[8] In a recent study, Stoll et al. reported that CK 19 expression was positive in only 50% of their 30 cases of dentigerous cysts with eight cases showing superficial expression while all the cases of odontogenic keratocysts were negative for CK 19.[2]
In the present study, 40% of the dentigerous cysts showed staining in all the layers of the lining epithelium while the remaining cases showed only partial staining. These results were similar to that of Gao et al.[8] but differed from that of Stoll et al.[2] in that all cases of dentigerous cysts were positive for CK 19. The pattern of CK 19 staining in dentigerous cysts in the present study were similar to that reported by Gao et al.[8] in that the specimens showed CK 19 staining either in the full thickness of the epithelium or in the superficial layers. CK 19 expression confined to the superficial layers of dentigerous cysts was also reported by Stoll et al. in eight of their 15 specimens of dentigerous cysts positive for CK 19.[2] In relation to odontogenic keratocysts, the 40% of the cases in the present study were negative for CK 19 while 60% showed positive staining in only few layers. This is different from that reported by Stoll et al.[2] where all cases of odontogenic keratocysts were negative. The staining pattern in which only a few layers showed expression for CK 19 is similar to that reported by Hormia et al.[10] and Gao et al.[8] However, the layers in which the staining was observed differed from that reported in some of the earlier studies. The staining of CK 19 in odontogenic keratocysts in the present study was mainly seen in the suprabasal layers and this pattern, although similar to that reported by Gao et al.,[8] was different from that reported by Hormia et al.,[10] where the odontogenic keratocysts had shown a positive staining for CK 19 mainly in the basal and parabasal layer of cells. However, it is to be understood that interpretation of CK staining is subjective depending on the evaluation by the individual pathologists. Moreover, CK 19 is only one of the several molecular markers, which may be beneficial in differentiating between the various odontogenic lesions. Hence, further investigations with a larger sample size to study the pattern of expression of other epithelial markers may also be beneficial in providing additional diagnostic parameters for the easy differentiation between the two lesions.
To conclude, the findings of the present study indicate and support the findings from some earlier studies that significant differences exist in the expression of CK 19 between the dentigerous cyst and odontogenic keratocyst, and these differences in CK 19 expression by these cysts may be utilized as an additional diagnostic tool in differentiating between these two lesions. However, it is to be understood that interpretation of CK staining is subjective depending on the evaluation by the individual pathologists. Moreover, CK 19 is only one of the several molecular markers, which may be beneficial in differentiating between the various odontogenic lesions. Hence, further investigations with a larger sample size to study the pattern of expression of other epithelial markers may also be beneficial in providing additional diagnostic parameters for the easy differentiation between the two lesions.
References
- Li TJ. The odontogenic keratocyst: A cyst, or a cystic neoplasm? J Dent Res 2011;90:133‑42.
- Stoll C, Stollenwerk C, Riediger D, Mittermayer C, Alfer J. Cytokeratin expression patterns for distinction of odontogenic keratocysts from dentigerous and radicular cysts. J Oral Pathol Med 2005;34:558‑64.
- MacDonald AW, Fletcher A. Expression of cytokeratin in the epithelium of dentigerous cysts and odontogenic keratocysts: An aid to diagnosis. J Clin Pathol 1989;42:736‑9.
- de Oliveira MG, Lauxen Ida S, Chaves AC, Rados PV, Sant’Ana Filho M. Immunohistochemical analysis of the patterns of p53 and PCNA expression in odontogenic cystic lesions. Med Oral Patol Oral Cir Bucal 2008;13:E275‑80.
- Wang YP, Liu BY. High expression of osteopontin and CD44v6 in odontogenic keratocysts. J Formos Med Assoc 2009;108:286‑92.
- Suyama Y, Kubota Y, Yamashiro T, Ninomiya T, Koji T, Shirasuna K. Expression of keratinocyte growth factor and its receptor in odontogenic keratocysts. J Oral Pathol Med 2009;38:476‑80.
- Bader BL, Magin TM, Hatzfeld M, Franke WW. Amino acid sequence and gene organization of cytokeratin no. 19, an exceptional tail‑less intermediate filament protein. EMBO J 1986;5:1865‑75.
- Gao Z, Mackenzie IC, Cruchley AT, Williams DM, Leigh I, Lane EB. Cytokeratin expression of the odontogenic epithelia in dental follicles and developmental cysts. J Oral Pathol Med 1989;18:63‑7.
- Maeda Y, Hirota J, Yoneda K, Osaki T. Immunohistochemical study of jaw cysts: Different existence of keratins in odontogenic and non‑odontogenic epithelial linings. J Oral Pathol Med 1990;19:289‑94.
- Hormia M, Ylipaavalniemi P, Nagle RB, Virtanen I. Expression of cytokeratins in odontogenic jaw cysts: Monoclonal antibodies reveal distinct variation between different cyst types. J Oral Pathol 1987;16:338‑46.
- Meara JG, Pilch BZ, Shah SS, Cunningham MJ. Cytokeratin expression in the odontogenic keratocyst. J Oral Maxillofac Surg 2000;58:862‑5.
- Gao Z, Mackenzie IC, Williams DM, Cruchley AT, Leigh I, Lane EB. Patterns of keratin‑expression in rests of Malassez and periapical lesions. J Oral Pathol 1988;17:178‑85.
- Moll R, Divo M, Langbein L. The human keratins: Biology and pathology. Histochem Cell Biol 2008;129:705‑33.
- Lindberg K, Rheinwald JG. Suprabasal 40 kd keratin (K19) expression as an immunohistologic marker of premalignancy in oral epithelium. Am J Pathol 1989;134:89‑98.
- Bosch FX, Ouhayoun JP, Bader BL, Collin C, Grund C, Lee I, et al. Extensive changes in cytokeratin expression patterns inpathologically affected human gingiva. Virchows Arch B Cell Pathol Incl Mol Pathol 1989;58:59‑77.
- van Sprundel RG, van den Ingh TS, Desmet VJ, Katoonizadeh A, Penning LC, Rothuizen J, et al. Keratin 19 marks poor differentiation and a more aggressive behaviour in canine and human hepatocellular tumours. Comp Hepatol 2010;9:4.
- Saleh HA, Jin B, Barnwell J, Alzohaili O. Utility of immunohistochemical markers in differentiating benign from malignant follicular‑derived thyroid nodules. Diagn Pathol 2010;5:9.
- Alix‑Panabières C, Vendrell JP, Slijper M, Pellé O, Barbotte E, Mercier G, et al. Full‑length cytokeratin‑19 is released by human tumor cells: A potential role in metastatic progression of breast cancer. Breast Cancer Res 2009;11:R39.
- Chang CC, Yang SH, Chien CC, Chen SH, Pan S, Lee CL, et al. Clinical meaning of age‑related expression of fecal cytokeratin 19 in colorectal malignancy. BMC Cancer 2009;9:376.
- Li N, Jian X, Hu Y, Xu C, Yao Z, Zhong X. Discovery of novel biomarkers in oral submucous fibrosis by microarray analysis. Cancer Epidemiol Biomarkers Prev 2008;17:2249‑59.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.