Dexmedetomidine: An Adjuvant Making Large Inroads into Clinical Practice
- *Corresponding Author:
- Dr. Sukhminder Jit Singh Bajwa
Department of Anesthesiology and Intensive Care, Gian Sagar Medical College and Hospital, Ram Nagar, Banur, House No-27-A, Ratan Nagar, Tripuri, Patiala - 147 001, Punjab, India.
E-mail: sukhminder_bajwa2001@ yahoo.com
Abstract
The introduction of newer more selective α−2 adrenergic agonist, dexmedetomidine has made a revolution in the field of anesthesia owing to its varied application. The aim of the current review is to highlight the various clinical and pharmacological aspects of dexmedetomidine in daily routine practice of anesthesiology and intensive care besides its potential role in other clinical specialties. This review of dexmedetomidine was carried out after searching the medical literature in Pubmed, Science direct, Scopus, Google scholar and various text books and journal articles using keywords anesthesia, dexmedetomidine, neurosurgery, pediatric surgery, regional dexmedetomidine, anesthesia, regional, neurosurgery, and pediatric surgery. Dexmedetomidine has made its application from a novel sedating agent in the intensive care unit to its use as an adjuvant in various regional anesthetic techniques because of its “cooperative sedation” without any respiratory depression. It has a favorable pharmacokinetic profile suitable to be used in the perioperative period to reduce the requirements of opioids and anesthetic drugs. There are few side‑effects of dexmedetomidine, which should always be kept in mind before choosing the patients for its use. The various side‑effects associated with dexmedetomidine include, but are not limited to hypotension, bradycardia, worsening of heart block, dry mouth, and nausea. However, large scale randomized controlled trials are still needed to establish various effects of dexmedetomidine and to clearly define its safety profile.
Keywords
α−2 adrenergic agonist, Dexmedetomidine, General anesthesia, Regional anesthesia
Introduction
The continued quest for a novel sedating agent for intensive care and need for drugs to blunt the stress response to the surgical stimulus has led to the increasing use of α-2 adrenergic agonists in these clinical settings. These drugs have a favorable pharmacological profile owing to their sympatholytic, sedative, analgesic, anxiolytic, and anesthetic drugs sparing effects.[1] Clonidine, which was introduced earlier as an anti-hypertensive was commonly used α-2 adrenergic agonist in various clinical scenarios including regional and general anesthesia.[2-4] However, with the introduction of newer more selective α-2 agonist dexmedetomidine, which has 8 times more affinity than clonidine for α-2 receptors is bringing newer concepts in anesthesia and intensive care practice. It was approved by the Food and Drug Administration (FDA) in 1999 for use in humans for short term sedation in intensive care unit. Initially used for sedation and analgesia in intensive care, its use has been extended to other various clinical situations as well as in regional anesthesia as a useful adjunct.[5]
Method of Literature Search
The review was performed after searching the full text and abstracts from the literature in PubMed, HINARI, Scopus, Science Direct, Ovid MEDLINE, and Google scholar and the following keywords combinations were used: Dexmedetomidine, α-2 adrenergic agonist, pharmacokinetics of dexmedetomidine, cardiac surgery, neurosurgery, pediatric surgery and regional anesthesia. Various text books of clinical pharmacology and anesthesiology as well as national and international anesthesia journals were also searched for full text articles related to dexmedetomidine.
Pharmacodynamics
Dexmedetomidine, an α-2 adrenergic agonist, acts by binding to G-protein coupled α-2 adrenergic receptors, which are found in central, peripheral, and autonomic nervous systems and also in various vital organs and blood vessels throughout the body.[6] There are three subtypes of these receptors namely α-2A, α-2B and α-2C each having different functions and activities. Dexmedetomidine is considered to have more affinity for α-2A and α-2C receptors as compared to clonidine.[7]
The site of action for sedative effects of dexmedetomidine is locus ceruleus and is mediated by hyperpolarization of noradrenergic neurons thus inhibiting noradrenaline release and inhibiting activity in descending medullospinal noradrenergic pathways.[8,9]
Analgesic effects are mainly mediated by α-2C and α-2A receptors present on the neurons of superficial dorsal horn in lamina II, by inhibiting the release of pro-nociceptive transmitters namely substance P and glutamate and by hyperpolarization of spinal interneurons.[10]
Activation of post-synaptic α-2 receptors lead to sympatholysis and results in hypotension and bradycardia; thus, helps in attenuating the stress response. Other useful actions of dexmedetomidine include decreased salivation, increased glomerular filtration, decreased intraocular pressure, decreased shivering threshold, decreased bowel motility, and decreased insulin release from pancreas.[11]
Pharmacokinetics
Dexmedetomidine has poor bioavailability due to extensive first pass metabolism; however, sublingual route has high bioavailability of about 84%.[12] It exhibits linear pharmacokinetics over a dose range of 0.2-0.7 μg/kg/h intravenous infusion. It is rapidly distributed with a volume of distribution being 118 l and has an elimination half-life of 2 h. It is 94% protein bound and does not displaces most of the protein bound drugs used commonly in anesthesia and intensive care. The context-sensitive half-life varies from 4 min for a 10 min infusion to 250 min for an 8 h infusion.
Dexmedetomidine undergoes complete biotransformation by glucoronidation and by cytochrome P-450 mediated aliphatic hydroxylation to inactive metabolites. These metabolites are excreted in the urine (95%) and in feces (4%). The dose needs to be adjusted in patients with hepatic failure towing to lower rates of metabolism.
Clinical Effects
Cardiovascular system
The effects of dexmedetomidine on blood pressure are biphasic with an initial transient rise with a reflex fall in heart rate brought about by stimulation of α-2B subtypes of receptors present in vascular smooth muscles. This is followed by fall in blood pressure and heart rate due to inhibition of central sympathetic outflow and stimulation of pre-synaptic α-2 receptors cause decreased release of nor-adrenaline leading to further fall in the blood pressure.[13,14] These hemodynamic effects; however, may be deleterious in patients with fixed stroke volume, on rate reducing drugs such as beta blockers, digitalis etc., and in hypovolemic patients.
Central nervous system
Dexmedetomidine causes a reduction in cerebral blood flow and cerebral metabolic demand of oxygen with a slight reduction in intracranial pressure. It has found to have neuroprotective effects by reducing the circulating and cerebral catecholamines; thus, reducing the excitotoxicity and improving the blood supply to the ischemic cerebral tissues. It also reduces the levels of glutamate, which is found to enhance the cellular brain injury especially in subarachnoid hemorrhage.[15]
Respiratory system
Dexmedetomidine does not have any depressant effects on respiratory function even at higher doses with no impairment of ventilation or gas exchange; however, may produce mild hypercapnia.[16,17] It is considered to be a good sedating agent with good cardiovascular stability; thus, facilitating weaning in patients on prolonged ventilatory support with failed previous attempts.
Endocrine and renal system
Dexmedetomidine causes suppression of stress response to surgery by activation of peripheral α-2 receptors and reducing the release of catecholamines. It is found to have no inhibitory effects on steroidogenesis when used for short term sedation by intravenous infusion.[18,19]
Adverse effects
The common side-effects include hypotension, bradycardia, dry mouth, nausea, desaturation, pulmonary edema, atelectasis etc., Long-term infusions of dexmedetomidine may result in up-regulation of receptors leading to the development of withdrawal syndrome on abrupt discontinuation manifesting as nervousness, agitation, headaches, and hypertensive crisis.[20]
Its use is not recommended in patients with advanced heart block and with ventricular dysfunction and it is classified as category C risk in pregnant patients.[6]
Clinical Utility of Dexmedetomidine
Dexmedetomidine is increasingly being used in various clinical situations. The clinical utility of this wonder drug is getting expanded with availability of more and more literature from various studies carried out throughout the globe. The major clinical role of dexmedetomidine in anesthesiology and intensive care practice, which has been established can be summarized as:
Sedation in Critically Ill Patients
Dexmedetomidine was initially approved by FDA for use as sedative agent in intensive care unit owing to its favorable properties of linear pharmacokinetic profile, short elimination half-life and no respiratory depression. It mimics the normal sleep pattern and thus keeps the patients calm, quiet but arousable and cooperative. It was initially approved for use for less than 24 h as an intravenous infusion, but recently studies have demonstrated its efficacy for use beyond 24 h.[21-23] In the maximizing efficacy of targeted sedation and reducing neurological dysfunction trial, it is reported that the use of dexmedetomidine intravenous infusion for 24-120 h results in earlier return of a delirium-free cognitive state with an increase in ventilator-free days. Compared to propofol, it has been found to be equally effective for sedation in intensive care with added advantage of minimal respiratory depression and maintenance of stable hemodynamics with easy arosability.[24]
Caution should be exercised in patients with hypovolemia, reduced ventricular functions, high degree of conduction blocks and in patients who are vasoconstricted, in whom its adverse-effects can be partially attenuated by lowering the initial bolus dose.[25]
Procedural Sedation
The role of dexmedetomidine as a sole agent for sedation in various minimally invasive procedures is fast emerging owing to its faster onset of action and fast recovery times.[26] It has been found to be a safe alternative to a benzodiazepine/opioid combination for a variety of procedures requiring monitored anesthesia care for its “cooperative sedation” and no respiratory depression.[27-31]
The effects of dexmedetomidine can also be reversed by an antagonist of α-2 receptors named “atipamezole” so its sedation can easily be titrated and can be reversed.[32]
Perioperative Use
Dexmedetomidine because of its anxiolytic, analgesic, sympatholytic, and sedative effects, has found its application in premedication, prevention of stress response to laryngoscopy, and prevention of emergence delirium.
Use as Premedicant
Dexmedetomidine have been found to be very effective as a premedicant before the institution of general anesthesia owing to its sedative, anxiolytic and sympatholytic effects and has been found to reduce oxygen consumption in intraoperative (8%) as well as post-operative (17%) periods.[33] It has been found to have good bioavailability when given through relatively non-invasive routes such as nasal or buccal. The buccal route in particular, have found to have better compliance and good absorption when given in a dose of 3-4 μg/kg about 1 h prior to surgery.[34,35]
Intranasal administration of dexmedetomidine have also been found to provide better sedation and facilitates better parental separation when compared to oral midazolam given in a dose of 1 μg/kg intranasally 1 h prior to surgery. It may provide good and safe sedative effects and may have benefits over transmucosal or rectal routes of administration.[36,37]
During intraoperative period
Dexmedetomidine, due to its sympatholytic effects, blunts the hyperdynamic response to laryngoscopy and surgery and maintains a stable hemodynamic profile.[38,39] [Table 1]. It also has been found to potentiate the effects of all the anesthetic agents namely intravenous and inhalational and have opioid sparing effects thereby reducing the doses required.[40-43] It can also help in reducing the oxygen requirements of the body and helps in prevention of intraoperative myocardial ischemia.[44,45]
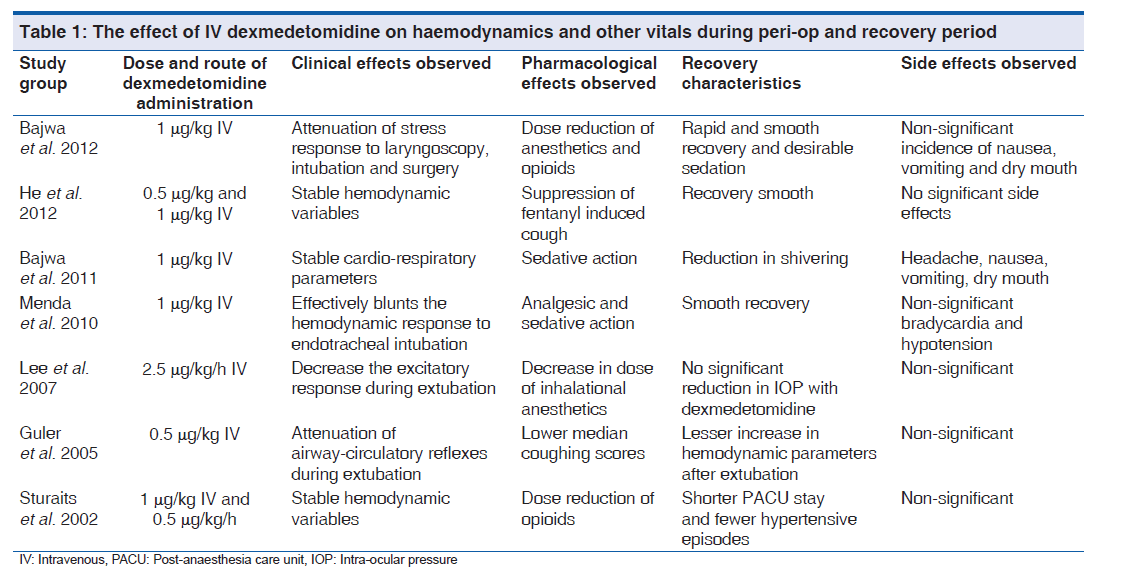
Dexmedetomidine have been reported to decrease the requirements of rocuronium in sevoflurane anesthesia and this effect may be attributed to the alteration of pharmacokinetics of rocuronium by dexmedetomidine.[46]
Dexmedetomidine has recently been utilized for facilitation of awake fiberoptic intubation in patients with compromised airway due to anatomical distortions and infections of upper airway. It provides a good sedation with analgesia with little or no respiratory depression as well as no effect on airway reflexes so that the patient remains calm and chances of aspiration are minimized.[47-49] Recently, it has been used in awake fiberoptic intubation without topical anesthesia of the upper airway as a sole sedative agent in a patient with documented allergy to local anesthetics.[50]
Dexmedetomidine have both sympatholytic effects and sparing of anesthetic effects, which make it an ideal for induction and maintenance of controlled hypotension in various surgeries minimizing the blood loss as well as providing optimal conditions for surgery such as spinal fusion surgery, endoscopic nasal, and sinus surgery and maxillofacial surgery.[51-53]
As postoperative adjunct and analgesic
Dexmedetomidine intravenous infusion can be continued during extubation as it has no respiratory depressant effects and it helps in blunting the stress response of extubation and the emergence delirium in some patients by keeping them calm and sedated. It also provides good post-operative analgesia and reduction in opioid requirements owing to its selective blockade of α-2A receptors.[54] It has also been shown to reduce the incidence of postoperative nausea and vomiting and helps in attenuating post-operative shivering; thus, reducing the post-operative oxygen metabolic demand, which can be very helpful in cardiac patients.[55]
Beneficial Role in Regional Anesthesia
Neuraxial anesthesia
Dexmedetomidine is highly lipophilic and thus is rapidly distributed in neural tissues and produces its antinociceptive effects by binding to α-2 receptors in spinal dorsal horn when used neuraxially.[56]
Epidural dexmedetomidine as adjuvant with local anesthetics prolongs the duration of sensory as well as motor blockade with more intense motor blockade and good postoperative analgesia.[57] The use of epidural dexmedetomidine as an adjuvant to local anesthetics when used in conjunction with general anesthesia have shown to lower intraoperative anesthetic requirements, improved oxygenation and prolonged post-operative analgesia [Table 2].[58-63] Few concerns have been raised from time to time with failure of breastfeeding after epidural anesthesia, but with epidural dexmedetomidine such concerns are out of question as studies are not available where dexmedetomidine is used as an adjunct in labor analgesia.[64] Dexmedetomidine has shown to be better when used as an adjuvant with ropivacaine in epidural anesthesia as compared to clonidine and fentanyl.[62,63]
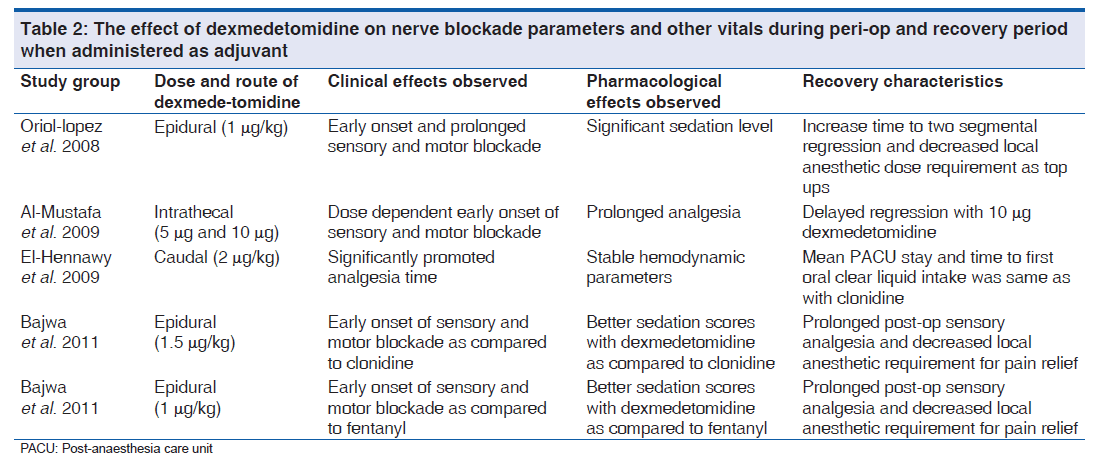
Intrathecal dexmedetomidine added to local anesthetic augments the sensory block, produces more intense motor blockade and prolongs the post-operative analgesia thus can decrease the dose of local anesthetics used.[60,65] Various doses have been tried intrathecally (3, 5, 10 μg) with favorable outcomes of prolongation of sensory/motor block with preserved hemodynamics; however, the prolonged motor block may not be ideal for ambulatory surgeries.[66] All the studies evaluating intrathecal dexmedetomidine are devoid of any neurological deficits, but some evidence of demyelination of oligodendrocytes in white matter have been seen in animal studies suggesting probable harmful effects of epidural dexmedetomidine on myelin sheath.[67] However, further clinical and pathological studies are required to safely establish the efficacy of intrathecal dexmedetomidine [Table 2].
Regional nerve blocks
In peripheral nerve blocks also dexmedetomidine has shown its efficacy in prolonging the duration of sensory block as well as prolongation of post-operative analgesia when used along with local anesthetic. Animal studies have validated the absence of neurotoxicity when directly applied to nerve models.[68,69] Dexmedetomidine have been successfully combined with various local anesthetics such as levobupivacaine, ropivacaine etc., with favorable results.[70] Furthermore, dexmedetomidine has been compared to clonidine as an adjuvant to the local anesthetic in peripheral nerve blocks like supraclavicular brachial plexus block and has been found to enhance the duration of sensory and motor blockade with prolongation of requirement of rescue analgesic.[70]
Intravenous regional anesthesia
Dexmedetomidine added to lignocaine in the intravenous regional blocks have shown to improve the block quality, decrease the tourniquet pain and prolongs the post-operative analgesia with minimal side-effects.[71,72]
Intra-articular infiltration
The peripheral analgesic effects of dexmedetomidine mediated through α-2A receptors have been utilized in direct intrarticular infiltration in arthroscopic knee surgeries with a prolongation of post-operative analgesia.[73]
Neuro-protective Role in Neurosurgical Procedures
Dexmedetomidine has an important role in neurosurgery by providing stable cerebral hemodynamics, blunting any rise in intracranial pressures during laryngoscopy and head pin insertion. It also helps in making the patients calm, comfortable and sedated, but easily arousable to perform neurocognitive and neuromotor examination as required in procedures like awake craniotomies, deep brain stimulation, minimally invasive endoscopic procedures, intraoperative imaging, stereotactic interventions etc.[74-76]
Studies have shown that when used for labor analgesia, dexmedetomidine is retained in the placental tissue owing to its high lipophilicity resulting in decreased transfer to the fetus leading to less chances of fetal bradycardia. Its use has been studied in parturients with failed epidural analgesia along with systemic opioids with a resultant good maternal anxiolysis, hemodynamic stability, and stimulation of uterine contractions.[77,78]
Role in Cardiac Surgery
Dexmedetomidine has been studied in vascular and cardiac surgery for its sedation and sympatholytic effects and has been found to be effective in maintenance of myocardial oxygen supply/demand ratio with consequent less chances of perioperative ischemia.[79] Studies have shown it to be helpful in managing patients undergoing mitral valve replacement surgery with co-existing pulmonary hypertension by reduction in pulmonary vascular resistance, pulmonary artery pressure, and pulmonary capillary wedge pressure.[6]
Use in Pediatric Population
As stated earlier, dexmedetomidine has been studied extensively as a premedicant in the pediatric population especially by nasal and buccal route with acceptable absorption and good compliance and better parental separation.[35,36] Few studies have been done on its role as an adjuvant in sedation of pediatric patients in critical care unit and during non-invasive procedures in radiology such as computed tomography and magnetic resonance imaging.[80]
Therapeutic Role in Opioid and Alcohol Withdrawal
Recently, dexmedetomidine have been shown to be an effective drug in opioid or benzodiazepine withdrawal by reducing the sympathetic outflow and noradrenergic stimulation caused by the withdrawal. This is mainly attributed to their blocking of α-2A receptors situated in the locus ceruleus.[81,82] It has been found to be helpful controlling the agitation in alcoholics after traumatic brain injury and thus helps in monitoring and allows serial neurotesting in these patients.[83]
Role in Cancer Pain
Dexmedetomidine has been studied as an adjuvant in intractable cancer pain and has been found to benefit in reduction of pain refractory to multiple treatment modalities.[84]
Newer Potential Uses
Few studies in animals have found a diuretic effect of dexmedetomidine by inhibition of antidiuretic action of vasopressin at the collecting duct and have also been found to attenuate radio contrast nephropathy by preserving cortical blood flow.[85] Recently, it has been found to be effective in controlling supraventricular and junctional tachyarrhythmias.[86]
Evidence Based Comparison with Other Drugs
Dexmedetomidine has been compared with propofol for sedation in intensive care patients and has been found to be equally efficacious with less incidence of respiratory depression, easy arousabilty and stable hemodynamic parameters.[24] Compared to a benzodiazepine and opioid combination, dexmedetomidine has been found to be more effective in providing sedation in procedures involving monitored anesthesia.[30,31]
Dexmedetomidine has been compared to oral midazolam through intranasal route as a premedicant in a dose of 1ug/ kg in the pediatric population and has been found to be more effective in providing sedation as well as good parental separation.[36] it has been compared to remifentanyl and esmolol for providing controlled hypotension in tympanoplasty surgery, which suggests it to be equally effective in reducing mucosal bleeding with less adverse effects and favorable recovery profile.[87]
Recently, dexmedetomidine has been compared to ketamine and placebo on emergence agitation after strabismus surgery in pediatric patients under sevoflurane anesthesia and was found to inhibit the postoperative emergence agitation, which was similar to the ketamine, but had a greater effect in reducing the post-operative nausea and vomiting when compared to ketamine.[88]
Dexmedetomidine used as an adjuvant to epidural local anesthetics has been compared to clonidine and fentanyl and has shown to shorten the onset of sensory and motor block as well as prolongs the post-operative sensory analgesia with reduced requirements of local anesthetics.[62,63] it has been shown to prolong post-operative sensory analgesia when used as an adjuvant to local anesthetic intrathecally as well as in peripheral nerve blocks when compared to fentanyl and clonidine.[66,70]
Recently, a comparison has been carried out between different infusion doses of dexmedetomidine (0.2 ug/ kg/h and 0.4 ug/kg/h) on sedation profile and was found have similar results; however, increasing the infusion dose delayed some recovery parameters.[89] A comparison of two different doses of dexmedetomidine (0.5 ug/kg and 1 ug/kg) to suppress hemodynamic changes to tracheal intubations showed that the higher dose was more effective when used as bolus.[90]
Conclusion
In conclusion, from the above discussion, it is clear that the newer α-2 adrenergic receptor blocker dexmedetomidine is a promising drug having numerous useful applications. It can be a very helpful drug in the armamentarium of an anesthesiologist having its use in perioperative care as well as in the treatment of chronic pain. Since, its introduction in the clinical practice, dexmedetomidine has come a long way with a new use being discovered every day, but still a lot of research has to be carried out with randomized controlled trials for its various effects.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Kemp KM, Henderlight L, Neville M. Precedex: Is it the future of cooperative sedation? Nursing 2008;38:7-8.
- Bajwa SJ, Bajwa SK, Kaur J, Singh A, Singh A, Parmar SS. Prevention of hypotension and prolongation of postoperative analgesia in emergency cesarean sections: A randomized study with intrathecal clonidine. Int J Crit Illn Inj Sci 2012;2:63-9.
- Bajwa SJ, Kaur J, Bajwa SK, Bakshi G, Singh K, Panda A. Caudal ropivacaine-clonidine: A better post-operative analgesic approach. Indian J Anaesth 2010;54:226-30.
- Bajwa SJ, Bajwa SK, Kaur J. Comparison of epidural ropivacaine and ropivacaine clonidine combination for elective cesarean sections. Saudi J Anaesth 2010;4:47-54.
- Takrouri MS, Seraj MA, Channa AB, el-Dawlatly AA, Thallage A, Riad W, et al. Dexmedetomidine in intensive care unit: A study of hemodynamic changes. Middle East J Anesthesiol 2002;16:587-95.
- Afsani N. Clinical application of dexmedetomidine. S Afr J Anaesthesiol Analg 2010;16:50-6.
- Fairbanks CA, Stone LS, Wilcox GL. Pharmacological profiles of alpha 2 adrenergic receptor agonists identified using genetically altered mice and isobolographic analysis. Pharmacol Ther 2009;123:224-38.
- Guo TZ, Jiang JY, Buttermann AE, Maze M. Dexmedetomidine injection into the locus ceruleus produces antinociception. Anesthesiology 1996;84:873-81.
- Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol 2008;21:457-61.
- Ishii H, Kohno T, Yamakura T, Ikoma M, Baba H. Action of dexmedetomidine on the substantia gelatinosa neurons of the rat spinal cord. Eur J Neurosci 2008;27:3182-90.
- Philipp M, Brede M, Hein L. Physiological significance of alpha (2)-adrenergic receptor subtype diversity: One receptor is not enough. Am J Physiol Regul Integr Comp Physiol 2002;283:R287-95.
- De Wolf AM, Fragen RJ, Avram MJ, Fitzgerald PC, Rahimi-Danesh F. The pharmacokinetics of dexmedetomidine in volunteers with severe renal impairment. Anesth Analg 2001;93:1205-9.
- Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology 1992;77:1134-42.
- Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg 2000;90:699-705.
- Bekker A, Sturaitis MK. Dexmedetomidine for neurological surgery. Neurosurgery 2005;57:1-10.
- Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care 2000;4:302-8.
- Hsu YW, Cortinez LI, Robertson KM, Keifer JC, Sum-Ping ST, Moretti EW, et al. Dexmedetomidine pharmacodynamics: Part I: Crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology 2004;101:1066-76.
- Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology 2000;93:382-94.
- Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth 2001;86:650-6.
- Morgan GE, Mikhail MS, Murray MJ. Preoperative medication in Clinical Anaesthesia. In: Morgan GE, Mikhail MS, Murray MJ, editors. 4th ed. New York: Mcgraw Hill; 2006. p. 248.
- Ruokonen E, Parviainen I, Jakob SM, Nunes S, Kaukonen M, Shepherd ST, et al. Dexmedetomidine versus propofol/ midazolam for long-term sedation during mechanical ventilation. Intensive Care Med 2009;35:282-90.
- Guinter JR, Kristeller JL. Prolonged infusions of dexmedetomidine in critically ill patients. Am J Health Syst Pharm 2010;67:1246-53.
- Gerlach AT, Murphy CV, Dasta JF. An updated focused review of dexmedetomidine in adults. Ann Pharmacother 2009;43:2064-74.
- Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth 2001;87:684-90.
- Manecke Jr. GR, Ingersoll-Weng E, Thistlethwaite PA. Dexmedetomidine and Asystole. Anesthesiology 2004;101:1480.
- Shukry M, Miller JA. Update on dexmedetomidine: Use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag 2010;6:111-21.
- Su F, Hammer GB. Dexmedetomidine: Pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf 2011;10:55-66.
- Mason KP. Sedation trends in the 21st century: The transition to dexmedetomidine for radiological imaging studies. Paediatr Anaesth 2010;20:265-72.
- Bekker AY, Basile J, Gold M, Riles T, Adelman M, Cuff G, et al. Dexmedetomidine for awake carotid endarterectomy: Efficacy, hemodynamic profile, and side effects. J Neurosurg Anesthesiol 2004;16:126-35.
- Ghali A, Mahfouz AK, Ihanamäki T, El Btarny AM. Dexmedetomidine versus propofol for sedation in patients undergoing vitreoretinal surgery under sub-Tenon’s anesthesia. Saudi J Anaesth 2011;5:36-41.
- Kulshrestha A, Bajwa SJ, Singh A, Kapoor V. Dexmedetomidine and fentanyl combination for procedural sedation in a case of Duchenne muscular dystrophy. Anesth Essays Res 2011;5:224-6.
- Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: A pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology 1998;89:574-84.
- Taittonen MT, Kirvelä OA, Aantaa R, Kanto JH. Effect of clonidine and dexmedetomidine premedication on perioperative oxygen consumption and haemodynamic state. Br J Anaesth 1997;78:400-6.
- Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol 2003;56:691-3.
- Sakurai Y, Obata T, Odaka A, Terui K, Tamura M, Miyao H. Buccal administration of dexmedetomidine as a preanesthetic in children. J Anesth 2010;24:49-53.
- Yuen VM, Hui TW, Irwin MG, Yuen MK. A comparison of intranasal dexmedetomidine and oral midazolam for premedication in pediatric anesthesia: A double-blinded randomized controlled trial. Anesth Analg 2008;106:1715-21.
- Yuen VM, Hui TW, Irwin MG, Yao TJ, Wong GL, Yuen MK. Optimal timing for the administration of intranasal dexmedetomidine for premedication in children. Anaesthesia 2010;65:922-9.
- Guler G, Akin A, Tosun Z, Eskitascoglu E, Mizrak A, Boyaci A. Single-dose dexmedetomidine attenuates airway and circulatory reflexes during extubation. Acta Anaesthesiol Scand 2005;49:1088-91.
- Bajwa SJ, Kaur J, Singh A, Parmar S, Singh G, Kulshrestha A, et al. Attenuation of pressor response and dose sparing of opioids and anaesthetics with pre-operative dexmedetomidine. Indian J Anaesth 2012;56:123-8.
- He L, Xu JM, Dai RP. Dexmedetomidine reduces the incidence of fentanyl-induced cough: A double-blind, randomized, and placebo-controlled study. Ups J Med Sci 2012;117:18-21.
- Menda F, Köner O, Sayin M, Türe H, Imer P, Aykaç B. Dexmedetomidine as an adjunct to anesthetic induction to attenuate hemodynamic response to endotracheal intubation in patients undergoing fast-track CABG. Ann Card Anaesth 2010;13:16-21.
- Lee YY, Wong SM, Hung CT. Dexmedetomidine infusion as a supplement to isoflurane anaesthesia for vitreoretinal surgery. Br J Anaesth 2007;98:477-83.
- Sturaitis M, Kroin J, Swamidoss C, Moric M. Effects of intraoperative dexmedetomidine infusion on hemodynamic stability during brain tumor resection. Anesthesiology 2002;98:A-310.
- Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology 2000;93:1345-9.
- Panda BK, Singh P, Marne S, PawarA, Keniya V, Ladi S, et al. A comparison study of dexmedetomidine vs clonidine for sympathoadrenal response, perioperative drug requirements and cost analysis. Asia Pac J Trop Dis 2012;2 (Suppl 2):S815-21.
- Memis D, Turan A, Karamanlioglu B, Seker S, Pamukcu Z. Dexmedetomidine reduces rocuronium dose requirement insevoflurane anaesthesia. Curr Anaesth Crit Care 2008;19:169-74.
- Maroof M, Khan RM, Jain D, Ashraf M. Dexmedetomidine is a useful adjunct for awake intubation. Can J Anaesth 2005;52:776-7.
- Grant SA, Breslin DS, MacLeod DB, Gleason D, Martin G. Dexmedetomidine infusion for sedation during fibreoptic intubation: A report of three cases. J Clin Anesth 2004;16:124-6.
- Boyd BC, Sutter SJ. Dexmedetomidine sedation for awake fiberoptic intubation of patients with difficult airways due to severe odontogenic cervicofacial infections. J Oral Maxillofac Surg 2011;69:1608-12.
- Madhere M, Vangura D, Saidov A. Dexmedetomidine as sole agent for awake fiberoptic intubation in a patient with local anesthetic allergy. J Anesth 2011;25:592-4.
- El-Gohary MM, Arafa AS. Dexmedetomidine as a hypotensive agent: Efficacy and hemodynamic response during spinal surgery for idiopathic scoliosis in adolescents. Egyp J Anaesth 2010;26:305-11.
- Ayoglu H, Yapakci O, Ugur MB, Uzun L, Altunkaya H, Ozer Y, et al. Effectiveness of dexmedetomidine in reducing bleeding during septoplasty and tympanoplasty operations. J Clin Anesth 2008;20:437-41.
- Richa F, Yazigi A, El hage C, Jebara S, Hokayem N, Antakly MC. Dexmedetomidine: An agent for controlled hypotension in maxilla-facial surgery. Eur J Anaesthesiol 2004;21:902-6.
- Grosu I, Lavand’homme P. Use of dexmedetomidine for pain control. F1000 Med Rep 2010;2:90.
- Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol 2012;28:86-91.
- Pertovaara A. Antinociception induced by alpha-2- adrenoceptor agonists, with special emphasis on medetomidine studies. Prog Neurobiol 1993;40:691-709.
- Salgado PF, Sabbag AT, Silva PC, Brienze SL, Dalto HP, Módolo NS, et al. Synergistic effect between dexmedetomidine and 0.75% ropivacaine in epidural anesthesia. Rev Assoc Med Bras 2008;54:110-5.
- Elhakim M, Abdelhamid D, Abdelfattach H, Magdy H, Elsayed A, Elshafei M. Effect of epidural dexmedetomidine on intraoperative awareness and post-operative pain after one-lung ventilation. Acta Anaesthesiol Scand 2010;54:703-9.
- Oriol-lopez SA, Maldonado-Sanchez KA, Hernandez- Bernal CE, Castelazo-Arredondo JA, Moctezuma RL. Epidural dexmedetomidine in regional anaesthesia to reduce anxiety. Revista Mexicana de Anaestesiologia 2008;31:271-77.
- Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J 2009;30:365-70.
- El-Hennawy AM, Abd-Elwahab AM, Abd-Elmaksoud AM, El-Ozairy HS, Boulis SR. Addition of clonidine or dexmedetomidine to bupivacaine prolongs caudal analgesia in children. Br J Anaesth 2009;103:268-74.
- Bajwa SJ, Arora V, Kaur J, Singh A, Parmar SS. Comparative evaluation of dexmedetomidine and fentanyl for epidural analgesia in lower limb orthopedic surgeries. Saudi J Anaesth 2011;5:365-70.
- Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth 2011;55:116-21.
- Bajwa SJ, Bajwa SK. Impact of epidural analgesia on breast feeding: A possible relation and the existing controversies. J Obstet Anaesth Crit Care 2012;2:57-9.
- Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand 2006;50:222-7.
- Al-Ghanem SM, Massad IM, Al-Mustafa MA, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures: A double blind controlled study. Am J Applied Sci 2009;6:882-7.
- Konakci S, Adanir T, Yilmaz G, Rezanko T. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol 2008;25:403-9.
- Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg 2010;111:1548-51.
- Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology 2008;109:502-11.
- Swami SS, Keniya VM, Ladi SD, Rao R. Comparison of dexmedetomidine and clonidine (a2 agonist drugs) as an adjuvant to local anaesthesia in supraclavicular brachial plexus block: A randomised double-blind prospective study. Indian J Anaesth 2012;56:243-9.
- Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology 2009;111:1111-9.
- Memis D, Turan A, Karamanlioglu B, Pamukçu Z, Kurt I. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg 2004;98:835-40.
- Ramadhyani U, Park JL, Carollo DS, Waterman RS, Nossaman BD. Dexmedetomidine: Clinical application as an adjunct for intravenous regional anesthesia. Anesthesiol Clin 2010;28:709-22.
- Paul S, Bhattacharjee DP, Ghosh S, Dawn S, Chatterjee N. Efficacy of intra-articular dexmedetomidine for postoperative analgesia in arthroscopic knee surgery. Ceylon Med J 2010;55:111-5.
- Tanskanen PE, Kyttä JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: A double-blind, randomized and placebo-controlled study. Br J Anaesth 2006;97:658-65.
- Frost EA, Booij LH. Anesthesia in the patient for awake craniotomy. Curr Opin Anaesthesiol 2007;20:331-5.
- Mack PF, Perrine K, Kobylarz E, Schwartz TH, Lien CA. Dexmedetomidine and neurocognitive testing in awake craniotomy. J Neurosurg Anesthesiol 2004;16:20-5.
- Abu-Halaweh SA, Al Oweidi AK, Abu-Malooh H, Zabalawi M, Alkazaleh F, Abu-Ali H, et al. Intravenous dexmedetomidine infusion for labour analgesia in patient with preeclampsia. Eur J Anaesthesiol 2009;26:86-7.
- Palanisamy A, Klickovich RJ, Ramsay M, Ouyang DW, Tsen LC. Intravenous dexmedetomidine as an adjunct for labor analgesia and cesarean delivery anesthesia in a parturient with a tethered spinal cord. Int J Obstet Anesth 2009;18:258-61.
- Talke P, Li J, Jain U, Leung J, Drasner K, Hollenberg M, et al. Effects of perioperative dexmedetomidine infusion in patients undergoing vascular surgery. The Study of Perioperative Ischemia Research Group. Anesthesiology 1995;82:620-33.
- Phan H, Nahata MC. Clinical uses of dexmedetomidine in pediatric patients. Paediatr Drugs 2008;10:49-69.
- Oschman A, McCabe T, Kuhn RJ. Dexmedetomidine for opioid and benzodiazepine withdrawal in pediatric patients. Am J Health Syst Pharm 2011;68:1233-8.
- Tang JF, Chen PL, Tang EJ, May TA, Stiver SI. Dexmedetomidine controls agitation and facilitates reliable, serial neurological examinations in a non-intubated patient with traumatic brain injury. Neurocrit Care 2011;15:175-81.
- Roberts SB, Wozencraft CP, Coyne PJ, Smith TJ. Dexmedetomidine as an adjuvant analgesic for intractable cancer pain. J Palliat Med 2011;14:371-3.
- Billings FT 4th, Chen SW, Kim M, Park SW, Song JH, Wang S, et al. alpha2-Adrenergic agonists protect against radiocontrast-induced nephropathy in mice. Am J Physiol Renal Physiol 2008;295:F741-8.
- Chrysostomou C, Shiderly D, Berry D. Dexmedetomidine, a novel agent for the acute treatment of supraventricular tachyarrhythmias after pediatric cardiac surgery. Crit Care Med 2007;8:A2.
- Turan G, Dincer E, Ozgultekin A, Uslu C, Ormanci F, Akgun N. Comparison of dexmedetomidine, Remifentanyl and Esmolol in controlled hypotensive anaesthesia. Internet J Anesthesiol 2008.
- Chen JY, Jia JE, Liu TJ, Qin MJ, Li W ×. Comparison of the effects of dexmedetomidine, ketamine, and placebo on emergence agitation after strabismus surgery in children. Can J Anaesth 2013;60:385-392.
- Kawaai H, Satoh J, Watanabe M, Kan K, Ganzberg S, Yamazaki S. A comparison of intravenous sedation with two doses of dexmedetomidine: 0.2 µg/kg/hr Versus 0.4 µg/kg/hr. Anesth Prog 2010;57:96-103.
- Sagiroglu A, Celik M, Orhon Z, Yüzer S, Sen B. Different doses of dexmedetomidine on controlling haemodynamic responses to tracheal intubation. Internet J Anesthesiol 2010.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.