Diagnosis, Prevention and Management of Postoperative Pulmonary Edema
- *Corresponding Author:
- Dr. Sukhminder Jit Singh Bajwa
House No-27-A, Ratan Nagar, Tripuri, Patiala, Punjab- 147 001, India.
E-mail: sukhminder_bajwa2001@yahoo.com
Citation: Bajwa SS, Kulshrestha A. Diagnosis,prevention and management of postoperative pulmonary edema. Ann Med Health Sci Res 2012;2:180-5.
Abstract
Postoperative pulmonary edema is a well‑known postoperative complication caused as a result of numerous etiological factors which can be easily detected by a careful surveillance during postoperative period. However, there are no preoperative and intraoperative criteria which can successfully establish the possibilities for development of postoperative pulmonary edema. The aims were to review the possible etiologic and diagnostic challenges in timely detection of postoperative pulmonary edema and to discuss the various management strategies for prevention of this postoperative complication so as to decrease morbidity and mortality. The various search engines for preparation of this manuscript were used which included Entrez (including Pubmed and Pubmed Central), NIH.gov, Medknow.com, Medscape.com, WebMD.com, Scopus, Science Direct, MedHelp.org, yahoo.com and google. com. Manual search was carried out and various text books and journals of anesthesia and critical care medicine were also searched. From the information gathered, it was observed that postoperative cardiogenic pulmonary edema in patients with serious cardiovascular diseases is most common followed by noncardiogenic pulmonary edema which can be due to fluid overload in the postoperative period or it can be negative pressure pulmonary edema (NPPE). NPPE is an important clinical entity in immediate post‑extubation period and occurs due to acute upper airway obstruction and creation of acute negative intrathoracic pressure. NPPE carries a good prognosis if promptly diagnosed and appropriately treated with or without mechanical ventilation.
Keywords
Acute respiratory distress syndrome, Cardiogenic pulmonary edema, Mechanical ventilation, NPPE, Postoperative pulmonary edema
Introduction
Postoperative pulmonary edema is a well‑known postoperative complication with little known etiology and mortality.[1‑4] Even the preoperative and intra‑operative criteria which can successfully establish the possibilities for development of postoperative pulmonary edema have been studied extensively without any conclusive results. However, several etiologic factors have been described which could possibly lead to postoperative pulmonary edema with a lot of evidence suggesting that most of these patients have preexisting heart diseases. Over administration of intravenous fluids during perioperative period may precipitate cardiac dysfunction which can prove to be fatal if progresses to ischemia.[5] Besides fluid overload, there are other pathogenic mechanisms which can lead to this serious clinical presentation.[6‑8] Neurogenic pulmonary edema is one such potential cause for the development of this pathology. Acute respiratory distress syndrome (ARDS) is considered to be an important differential diagnosis especially in the critically ill and polytrauma patients. Other clinical conditions like head injury, hyponatremia, adrenal tumors, sepsis, pneumonitis, and so on are also implicated in causation of postoperative pulmonary edema. More challenging aspects associated with postoperative pulmonary edema include the exact diagnosis of its varied presentation and the resultant clinical manifestations. The basic understanding of the pathophysiology as well as the appropriate therapeutic interventions is the cornerstone for its successful treatment.
The scenario is immensely significant in the clinical settings of developing nations like Asian and African countries where majority of the surgical centers do not have attached intensive care services to handle such devastating complications. The role of noninvasive mechanical ventilation, continuous positive airway pressure (CPAP), and biphasic positive airway pressure (BIPAP) has gained immense popularity over the last two decades while treating the patients with pulmonary edema. However, its role in the health set‑up of developing nations will be difficult to define as majority of the peripheral health centers and private clinics may not possess the costly equipment and services of trained anesthesiologist. As such there is an acute need for reviewing the various clinical aspects associated with postoperative pulmonary edema in these nations in an attempt to decrease the morbidity and mortality arising thereof from this pathological entity. The present review is focused on identification of possible etiology, pathology, and appropriate management of postoperative pulmonary edema. The various search engines for preparation of this manuscript were used which included Entrez (including Pubmed and Pubmed Central), NIH.gov, Medknow. com, Medscape.com, WebMD.com, Scopus, Science Direct, MedHelp.org, yahoo.com and google.com. Manual search was carried out and various text books and journals of anesthesia and critical care medicine were also searched.
Materials and Methods
The various search engines for preparation of this manuscript were used which included Entrez (including Pubmed and Pubmed Central), NIH.gov, Medknow.com, Medscape.com, WebMD.com, Scopus, Science Direct, MedHelp.org, yahoo. com and google.com. Manual search was carried out and various text books and journals of anesthesia and critical care medicine were also searched.
Etiology
Though it is extremely difficult to identify the exact etiological factors but on the basis of present evidence these causative factors can be classified to some extent. The main etiologic factors may be divided into following major categories.
Cardiogenic pulmonary edema
This is the most important and most common cause of postoperative pulmonary edema in patients with preexisting cardiac diseases. Myocardial infarction is the most common finding in these patients resulting in left ventricular dysfunction and increased hydrostatic pressure in pulmonary circulation leading to leakage of fluid into the interstitium. Any undue stress during the surgical and anesthetic procedure can be a potential cause, especially the stress response associated with intubation, major handling of the viscera, and extubation response.[9,10] The other causes can be cardiac arrhythmias and congestive cardiac failure as cardiac patients are highly prone to develop these complications.
Noncardiogenic pulmonary edema
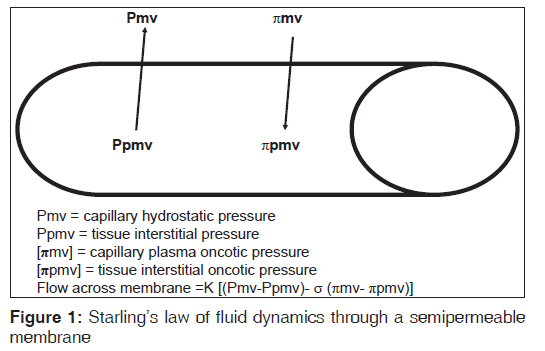
The postoperative pulmonary edema can develop in patients without any known cardiac disease as well with any underlying pathology. The etiology of this clinical condition is governed by starling’s law which has described the flow dynamics through the semi permeable membrane [Figure‑1]:
Q = K [(Pmv‑Ppmv)‑σ (πmv‑πpmv)]
where Q is the flow of fluid through membrane, K is filtration coefficient, Pmv is hydrostatic pressure in the pulmonary capillaries, Ppmv is the interstitial hydrostatic pressure, πmv oncotic pressure in the pulmonary capillaries, and πpmv is the interstitial oncotic pressure.
[σ] is the reflection coefficient which is used as a measure to correct for the ineffectiveness of some of the oncotic pressure gradient.
The normal fluid homeostasis in lungs is maintained by a good balance of filtration and absorption mechanisms between pulmonary capillaries and the draining lymphatics.[11] Any disturbance in these mechanisms can induce a state of pulmonary edema leading to respiratory failure.
Neurogenic pulmonary edema
The clinical manifestations in this of pulmonary edema can be attributed to disturbed autonomic nervous system with resultant exaggerated sympathetic discharge thereby raising the pulmonary capillary pressure and extravasations of fluid into pulmonary tissues. [12,13] The factors responsible for this pathological entity may include but are not limited to the following:
• Postoperative hyponatremic encephalopathy [6]
• Head trauma [7]
• Phaeochromocytoma [8]
• Air embolism.[14]
Fluid overload
Pulmonary edema can be extremely fatal if an early diagnosis is not made and treated appropriately. Majority of times, it may be the initial clinical manifestation of overenthusiastic fluid administration in certain diseased states such as sepsis, third space sequestration, excessive intraoperative hemorrhage, resuscitation during trauma and so on.[15‑18] It can also be secondary to acute renal failure in postoperative period or can result from excessive fluid intake.
The excessive amount of fluids in the postoperative period are usually given to replace the various “losses” during the surgical period such as continuing “third space losses”, evaporative losses, blood loss and insensible losses. This replacement of fluids is usually based on approximations of fluid requirement and clinical monitoring of various hemodynamic parameters and urinary output.[19,20] The quantity of fluid required to induce pulmonary edema in postoperative patients who do not have serious cardiovascular, hepatic or renal disorders depends upon age, body weight, tissue turgor, cardiovascular, renal, and pulmonary functions, plasma vasopressin levels, plasma proteins, and the volume of the “third space”.[2] A gain in fluid above 20% of the total body water has been termed as fluid overload [9] and it has been documented in the literature that pulmonary edema can occur within initial 36 hours postoperatively when the net fluid retention exceeds 67 ml/ kg/ day in absence of serious associated medical conditions.[21] The exact amount of fluids can be guided by monitoring of central venous pressures in postoperative patients with no cardiovascular or renal diseases and by the use of pulmonary capillary wedge pressures in patients with significant cardiovascular disease, so the fluid and electrolyte balance in early postoperative period is an essential factor in prevention of postoperative pulmonary edema.
Anaphylaxis causing pulmonary edema
This type of clinical entity is very difficult to diagnose as majority of times the patient seems to be exposed to multiple known and unknown etiological factors. The accumulation of fluid in the pulmonary tissues is mainly an end result of allergic reaction during the peri‑operative period. Most commonly implicated agents include antibiotics, anesthetic agents including ketamine, analgesics, neuro‑muscular blockers, adjuvant drugs or occasionally an allergy to latex.[22] The clinical presentation may include a sudden onset with bronchospasm, swelling, urticaria, rash, and/or disturbed hemodynamic parameters. The hypotension and tachycardia are predominantly seen in majority of the patients as they are under the influence of anesthesia. The diagnosis can be made either by exclusion or getting the levels of histamine and trypatse done. Ketamine, usually considered a safe drug, has recently been found to cause unusual anaphylactic reaction.[23,24]
Acute lung injury
Numerous etiologies including sepsis, trauma, burns, blood transfusions, aspiration, and chemical inhalation and so on can cause acute damage to the vascular endothelium in the pulmonary tissue and resulting in pulmonary edema. The clinical presentation may be in the form of acute lung injury or acute respiratory distress syndrome which may require ventilatory support till healing of the pulmonary tissue. However, the diagnosis of acute lung injury is often confused with cardiogenic pulmonary edema.
Negative pressure pulmonary edema
NPPE is basically a clinical entity which can be established by diagnosis of exclusion. It was first described in dogs by Moore [25] and was also reported in two children with acute upper airway obstruction by Capitanio et al.[26] NPPE is a pathophysiological syndrome which results due to acute development of negative intrathoracic pressure generated during spontaneous respiratory efforts against an obstructed upper airway. This is a postoperative catastrophic complication for which it is extremely difficult to define the possible etiological factors. As such, the appropriate management is focused on early identification and possible elective ventilation. The pathophysiological changes associated with it include:
1. Damage to pulmonary capillaries with fluid shift to the interstitium of the lungs with an increase in the transpulmonary blood volume. Due to marked negative intrathoracic pressure (−50 to −100 cmH2O), there is an increase in venous return to heart with subsequent elevation of ventricular end‑diastolic volumes and thus the left ventricular end‑diastolic pressures are elevated leading to formation of pulmonary edema.[27‑29]
2. The hypoxemia due to upper airway obstruction leads to increased pre‑ and postcapillary pulmonary vascular resistance in a nonuniform manner thus elevating the pulmonary vascular resistance and a hyper‑adrenergic state which precipitates pulmonary edema.[30] Not only do these mechanisms lead to fluid shift from pulmonary capillaries to perimicrovascularinterstitium but also cause disruption of alveolar capillary membrane.
This type of pulmonary edema due to acute upper airway obstruction is termed as type I negative pressure pulmonary edema (NPPE)
3. The changes in chronic airway obstruction are mainly due to sudden disappearance of autopositive end expiratory pressure (PEEP) and thus a sudden return of chronically elevated lung pressures. This generates a negative intrapulmonary pressure causing transudation of fluid in lung interstitium.
This type of pulmonary edema in chronic obstruction is termed as type II negative pressure pulmonary edema (NPPE).[31]
The overall incidence of NPPE is less than 0.1% in all surgeries performed under general anesthesia [32,33] while the incidence of development of pulmonary edema in acute upper airway obstruction (type I NPPE) ranges from 9.6‑12% and that in chronic airway obstruction (type II NPPE) is 44%.[34]
Type I NPPE is typically seen in young, healthy and athletic individuals with a slight male preponderance while type II NPPE is seen in extremes of ages.[31]
Etiology
The most common cause of NPPE remains postextubation laryngospasm.[35] Other causes of obstruction of upper airway leading to NPPE include the following:
• Hanging
• Laryngeal tumor
• Strangulation
• Sleep apnea
• Biting of endotracheal tube while intubated [36]
• Croup and epiglottitis especially in children [37]
• Administration of muscle relaxant at the beginning of an inhalational induction of anesthesia caused by premature paralysis of glossal muscles before diaphragm [38]
• Following aspiration of pneumothorax or massive pleural effusion
Clinical presentation
The clinical picture is similar irrespective of the cause of pulmonary edema while a history of recent extubation after anesthesia usually precedes the development of NPPE. The onset of NPPE may vary from few minutes to several hours (up to 30 hours) following extubation or relief of obstruction. [39] Type I NPPE usually presents with acute respiratory distress with signs of tachypnea, tachycardia, pink frothy pulmonary secretions, progressive oxygen desaturation and rales on chest auscultation.
Chest radiograph shows diffuse interstitial and alveolar infiltrates. Computed tomography of chest shows a preferential central and nondependent distribution of ground glass attenuation.[40]
Management
The management of postoperative pulmonary edema usually is aimed at treatment of the underlying cause. Majority of patients give good results with conservative and symptomatic treatment but few do require intubation and initiation of mechanical ventilation with application of positive end expiratory pressure. The cardiogenic pulmonary edema responds to the therapy directed towards the cardiac event causing pulmonary edema while that due to fluid overload usually responds to fluid restriction and diuretic therapy. Diuretics may be required to remove excess intrapulmonary fluid but should always be given after correcting fluid status of the patient.[28] However, a few researchers have claimed their role to be insignificant and consider administration of diuretics as un‑necessary part of the treatment.[41,42] The increased fluid accumulation during anaphylactic reaction requires administration of steroids and β agonists. The rapid regression of pulmonary edema on administration of β agonists occurs due to increased active cation transport.[43]
Noninvasive ventilatory support has gained immense popularity in the recent past and has replaced the traditional invasive intubation and ventilation strategies throughout the globe for the treatment of respiratory failure. The role of noninvasive ventilation in treatment of pulmonary edema can be significant as it drastically reduces the increased work of breathing and thereby preventing muscle fatigue. Apart from this benefit, noninvasive ventilation may be able to reduce the ventricular afterload, helps in better recruitment of alveoli as well as causes minimal disturbances of the hemodynamic parameters.[44,45] The incidence of ventilator associated pneumonias as well as hospital stay of the patient can possibly get reduced by adoption of noninvasive ventilation strategies ultimately resulting in decreased morbidity and mortality.[45,46]
Most cases of diagnosed NPPE respond promptly to appropriate therapy which is usually aimed at reversing hypoxia and removal of excess fluid from the lung interstitium. Patient might require early intubation and institution of mechanical ventilation if extubated while already intubated patients will need positive pressure ventilation with positive end expiratory pressure (PEEP) to maintain oxygen saturation.[41]
The resolution of pulmonary edema usually occurs within 3‑12 hours of institution of appropriate therapy with complete resolution of chest radiographic findings. However, in a few cases the complete resolution may take up to 12‑48 hours. The patients have good prognosis if promptly diagnosed and appropriate treatment instituted. However, a significant complication rate does exist and is generally attributed to a delay in diagnosis.[47,48]
Conclusion
In conclusion, postoperative pulmonary edema remains an important and fairly common complication which can easily be prevented by careful vigilance of the clinicians involved in postoperative care of patients. Negative pressure pulmonary edema (NPPE) is a type of pulmonary edema where a prompt diagnosis and early treatment can significantly reduce the complication rate. Importantly, in centers with no advanced postoperative care units or ICU facilities, development of such complications warrants immediate transfer of patients to a centre with available intensive care facilities so as to decrease the morbidity and mortality associated with such complications.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- van Hoozen BE, van Hoozen CM, Alberton TE. Pulmonary considerations and complications in the neurosurgical patient: Pulmonary edema. In: Youmans JR, editor. Neurological surgery. Philadelphia, PA: WB Saunders, 1996. p. 624-6.
- Roth E, Lax LC, Maloney JV Jr. Ringer?s lactate solution and extracellular fluid volume in the surgical patient: a critical analysis. Ann Surg 1969;169:149-64.
- Finn JC, Rosenthal MH. Pulmonary edema in trauma and critically ill patients. Semin Anesth 1989;8:265-74.
- Khuri SF, Daley J, Henderson W, Barbour G, Lowry P, Irvin G, et al. The National Veterans Administration Surgical RiskStudy: Risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg 1995;180:519-31.
- Mangano, DT, Browner, WS, Hollenberg, M, London MJ, Tubau JF, Tateo IM, et al. Association of perioperative myocardial ischemia with cardiac morbidity and mortality in men undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. N Engl J Med 1990;323:1781-8.
- Ayus JC, Arieff AI. Pulmonary complications of hyponatremic encephalopathy: Noncardiogenic pulmonary edema and hypercapnic respiratory failure. Chest 1995;107:517-21.
- Pandor A, Goodacre S, Harnan S, Holmes M, Pickering A, Fitzgerald P, et al. Diagnostic management strategies for adults and children with minor head injury: A systematic review and an economic evaluation. Health Technol Assess. 2011;15:1-202.
- Suga K, Tsukamoto K, Nishigauchi K, Kume N, Matsunaga N, Hayano T, et al. Iodine-123-MIBG imaging in pheochromocytoma with cardiomyopathy and pulmonary edema. J Nucl Med 1996;37:1361-4.
- Lowell JA, Schifferdecker C, Driscoll DF, Benotti PN, Bistrian BR. Postoperative fluid overload: Not a benign problem. Crit Care Med 1990;18:728-33.
- Katz AM. Cardiomyopathy of overload. A major determinant of prognosis in congestive heart failure. N Engl J Med 1990;322:100-10.
- Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev 2002;82:569-600.
- Johnston SC, Darragh TM, Simon RP. Postictal pulmonary edema requires pulmonary vascular pressure increases. Epilepsia 1996;37:428-32.
- West JB, Mathieu-Costello O. Stress failure of pulmonary capillaries: Role in lung and heart disease. Lancet 1992;340:762-7.
- Malik AB. Mechanisms of neurogenic pulmonary edema. Circ Res 1985;57:1-18.
- Dolinski SY, MacGregor DA, Scuderi PE. Pulmonary hemorrhage associated with negative-pressure pulmonary edema. Anesthesiology 2000;93:888-90.
- Ware LB, Fremont RD, Bastarache JA, Calfee CS, Matthay MA. Determining the etiology of pulmonary oedema by the oedema fluid-to-plasma protein ratio. Eur Respir J2010;35:331-7.
- Paul RE, George G. Fatal non-cardiogenic pulmonary oedema after intravenous non-ionic radiographic contrast. Lancet 2002;359:1037-8.
- Baumann A, Audibert G, McDonnell J, Mertes PM. Neurogenic pulmonary edema. Acta Anaesthesiol Scand 2007;51:447-55.
- Rosenthal MH, Arieff AI. Fluid and electrolyte therapy in critically ill patients and those who are pre-, post-or intraoperative. In: Arieff AI, DeFronzo RA, editors. Fluid, electrolyte and acid-base disorders. New York, NY: Churchill Livingstone; 1995. p. 597-632.
- Shires GT, Shires GT, Lowry SF. Fluid, electrolyte, and nutritional management of the surgical patient. In: Schwartz SI, Shires GT, Spencer FC, editors. Principles of surgery. New York, NY: McGraw-Hill; 1994. p. 61-93.
- Arieff AI. Fatal postoperative pulmonary edema: Pathogenesis and literature review. Chest 1999;115:1371-7.
- Dewachter P, Mouton-Faivre C, Emala CW. Anaphylaxis and anesthesia: Controversies and new insights. Anesthesiology 2009;111:1141-50.
- Kolawole IK. Misuse of Ketamine. The Nig J Surg Res 2001;3:175-80.
- Nwasor EO, Mshelbwala PM. An unusual reaction to ketamine in a child. Ann Nigerian Med 2010;4:28-30.
- Moore RL. The response to respiratory resistance: A comparison of the effects produced by the partial obstruction in the inspiratory and the expiratory phases of respiration. J Exp Med 1927;1065-80.
- Capitanio MA, Kirkpatrick JA. Obstruction of the upper airways in children as reflected on the chest radiograph.Radiology 1973;167:159-61.
- Kulka PJ, Issel R, Wiebalck A, Strumpf M, Gehling M. Delayed negative pressure pulmonary edema. Anaesthesist 2003;52:132-6.
- Newton-John H. Pulmonary oedema in upper airway obstruction. Lancet 1977;2:510.
- Fremont RD, Kallet RH, Matthay MA, Ware LB. Postobstructive pulmonary edema: A case for hydrostatic mechanisms. Chest 2007;131:1742-6.
- Sarnoff SJ, Burglund E, Sarnoff LC. Neurohemodynamics of pulmonary edema. III. Estimated changes in pulmonary blood volume accompanying systemic vasoconstriction and vasodilation. J Appl Physiol 1953;5:367-74.
- van Kooy MA, Gargiulo RF. Postobstructive pulmonary edema. Am Fam Physician 2000;62:401-4.
- Patton WC, Baker CL Jr. Prevalence of negative-pressure pulmonary edema at an orthopaedic hospital. J South Orthop Assoc 2000;9:248-53.
- Deepika K, Kenaan CA, Barrocas AM, Fonseca JJ, Bikazi GB. Negative pressure pulmonary edema after acute upper airway obstruction. J Clin Anesth 1997;9:403-8.
- G o l d e n b e r g J D , P o r t u g a l L G , W e i n g B L , Weingarten RT. Negative -pressure pulmonary edema in otolaryngology patient. Otolaryngol Head Neck Surg 1997;117:62-6.
- Mehta VM, Har-El G, Goldstein NA. Postobstructive pulmonary edema after laryngospasm in the otolaryngology patient. Laryngoscope 2006;116:1693-6.
- Liu EH, Yih PS. Negative pressure pulmonary oedema caused by biting and endotracheal tube occlusion--a case for oropharyngeal airways. Singapore Med J 1999;40:174-5.
- Bonadio WA, Losek JD. The characteristics of children with epiglottitis who develop the complication of pulmonary edema. Arch Otolaryngol Head Neck Surg 1991;117:205-7.
- Warner LO, Martino JD, Davidson PJ, Beach TP. Negative pressure pulmonary oedema: A potential hazard of muscle relaxants in awake infants. Can J Anaesth 1990;37:580-3.
- Glasser SA, Siler JN. Delayed onset of laryngospasm-induced pulmonary edema in an adult outpatient. Anesthesiology 1985;62:370-1.
- Cascade PN, Alexander GD, Mackie DS. Negative-pressure pulmonary edema after endotracheal intubation. Radiology 1993;186:671-5.
- Koh MS, Hsu AA, Eng P. Negative pressure pulmonary oedema in the medical intensive care unit. Intensive Care Med 2003;29:1601-4.
- Bhaskar B, Fraser JF. Negative pressure pulmonary edema revisited: Pathophysiology and review of management. Saudi J Anaesth 2011;5:308-13.
- Matthay MA, Fukuda N, Frank J, Kallet R, Daniel B,Sakuma T. Alveolar epithelial barrier: Role in lung fluid balance in clinical lung injury. Clin Chest Med 2000;21:477-90.
- Pelosi P, Jaber S. Noninvasive respiratory support in the perioperative period. Curr Opin Anaesthesiol 2010;23:233-8.
- Jaber S, Chanques G, Jung B. Postoperative noninvasive ventilation. Anesthesiology 2010;112:453-61.
- Goldenberg JD, Portugal LG, Wenig BL, Weingarten RT.Negative-pressure pulmonary edema in the otolaryngology patient. Otolaryngol Head Neck Surg 1997;117:62-6.
- Perez RO, Bresciani C, Jacob CE, Perez CG, Coser RB, Honda LF, et al. Negative pressure post-extubation pulmonary edema complicating appendectomy in a young patient. Curr Surg 2004;61:463-5.
- Louis P, Fernandes R. Negative pressure pulmonary edema. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2002;93:4-6.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.