Diagnostic Accuracy of Urine Microalbumin and Serum Uric Acid: A Case-control Study of Patients with Preeclampsia in the Komfo Anokye Teaching Hospital, Kumasi, Ghana
2 Department of Physiology, School of Medical Sciences, KNUST, Ghana
3 Department of Obstetrics and Gynecology, School of Medical Sciences, KNUST, Ghana
4 Department of Medical Laboratory Technology, School of Allied Health Sciences, UCC, Ghana, Email: rephraim@ucc.edu.gh
5 Department of Molecular Medicine, School of Medical Sciences, KNUST, Ghana
Citation: Ephraim M, et al. Diagnostic Accuracy of Urine Microalbumin and Serum Uric Acid; A Case-control Study of Patients with Preeclampsia in the Komfo Anokye Teaching Hospital, Kumasi. Ann Med Health Sci Res. 2018;8:77-83
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: There is increasing prevalence of preeclampsia coupled with the need to identify and institutionalize more sensitive diagnostic tools for preeclampsia. This study evaluated the diagnostic accuracy of urine microalbumin and serum uric acid as early markers of preeclampsia among Ghanaian women attending antenatal care at the Komfo Anokye Teaching Hospital (KATH). Methods: A case-control study was conducted among pregnant women at the Obstetrics and Gynaecology (O&G) department of the KATH, Kumasi-Ghana from October 2011 to May 2012. One hundred and twenty-three (123) participants were recruited for this study after written informed consent was obtained. Socio-demographic characteristics, medical history and previous obstetric history was obtained through medical records of the eligible participants. Blood pressure and anthropometrics were measured according to standard procedure; urine samples were collected for estimation of spot urine protein and microalbuminuria; and venous blood sample was taken for biochemical analysis and platelet count. Results: A significant positive linear correlation was observed between spot urine protein and urine microalbumin (r=0.324, p=0.006). A negative linear correlation was observed between uric acid and spot urine micro albumin (r=0.033, p=0.786). A urinary micro albumin value of 75.45 mg/g was identified as the best threshold to detect a spot urine protein of > +2 with a sensitivity of 92.7% and a specificity of 80.0%, PPV of 81.03% and NPV of 33.3%. Area under the curve = 0.835; asymptomatic p-value of 0.0001 at 95% CI (0.678-0.991). In contrast, serum uric acid level of 263.5 mg/g was identified as the best cut-off point to detect a spot urine protein of > +2 with sensitivity and specificity of 89.1% and 33.3% respectively (PPV of 77.2% and NPV) of 20.8%. Area under the curve = 0.552; asymptotic p-value of 0.538 at 95% CI (0.364-0.740). Conclusion: Urine levels of microalbumin, as a measure of proteinuria are elevated in preeclamptics and can be used in place of spot macro protein estimation to diagnose preeclampsia especially in the early stages.
Keywords
Preeclampsia; Microalbuminuria; Uric acid; Proteinuria
Abbreviations
KATH: Komfo Anokye Teaching Hospital; O&G: Obstetrics And Gynaecology; NPV: Negative Predictive Value; PPV: Positive Predictive Value; BMI: Body Mass Index; SMS: School Of Medical Sciences; KNUST: Kwame Nkrumah University Of Science And Technology; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure; ROC: Receiver Operator Characteristic; AST: Aspartate Transaminase; Alanine Transaminase; ALK PHOS: Alkaline Phosphatase; WHR: Waist To Hip Ratio; ALB: Albumin
Introduction
Preeclampsia is defined as a multisystem ailment of unknown etiology depicted by the development of elevated blood pressure to the level of 140/90 mmHg or more with proteinuria induced by pregnancy after 20 weeks of pregnancy in a previously normotensive and non-proteinuria pregnant woman. [1,2] In preeclampsia, there is elevated levels of serum uric acid due to reduced glomerular filtration rate, increased reabsorption and decreased secretion of serum uric acid, [3-5] proteinuria, high blood pressure and edema. Therefore, measurement of uric acid has been considered as a part of a panel of tests in women with preeclampsia to observe the severity of the disease to help with the management of the patients. [6,7]
According to Komfo Anokye Teaching Hospital (KATH) Annual Reports (2009 and 2013), Pre-eclampsia/eclampsia topped the first ten (10) causes of the admissions in the Directorate of Obstetrics and Gynaecology. In addition, preeclampsia/ eclampsia was the major cause of maternal mortality spanning the period 2009 to 2013 (Biostatistics unit, O & G, KATH, 2013). There are many options for diagnosis of proteinuria, including urinary dipstick testing, urinary protein: creatinine ratio, and various timed urine collections (most commonly, 24- hour urine). Most studies have focused on methods that best match the quantification of urinary protein by 24-hour urine collection, considered to be the gold standard. However, a 24- hour urine collection is time-consuming, inconvenient, and often not completed. [8] Also, the urinary protein: creatinine ratio has been accepted for diagnosis by the International and Australasian pregnancy hypertension societies.
Urinary dipstick testing is inexpensive, easy, and widely used. Its usefulness is uncertain for screening either women with hypertension or those who are at increased risk of preeclampsia. Hence, more information on the determination of proteinuria using other measures of proteinuria like microalbuminuria is needed before clinical use of the urinary microalbumin can be recommended. The increasing prevalence of hypertensive disorders of pregnancy, coupled with the need to identify and institutionalize more sensitive diagnostic tools has necessitated this study. In the light of the afore-mentioned, this study sought to determine the diagnostic accuracy of urine microalbumin and serum uric acid in patients with preeclampsia at the Komfo Anokye Teaching Hospital (KATH), Kumasi. The findings of this study will help to identify more sensitive markers that will help in early diagnosis of preeclampsia.
Materials and Methods
Study design/area
This non-randomized case-control study was conducted at the Obstetrics and Gynaecology (O&G) department of the Komfo Anokye Teaching Hospital (KATH) the major specialist and referral centre for the northern parts of the country from October 2011 to May 2012.
Ethical considerations
The participation of the respondents who are all indigenes of Ghana was voluntary and written informed consent was obtained from each of them. The study was approved by the Committees on Human Research Publication and Ethics, SMS/ KNUST and the Research Directorate of KATH.
Inclusion and exclusion criteria
Pregnant women with gestational age 20 weeks or more were considered eligible to participate in this study. For controls, we enrolled women in good health, normotensive and without dipstick proteinuria. Participants with elevated blood pressure (≥140/90 mmHg) with dipstick proteinuria (≥”+”) were enrolled as cases. Pregnant women with chronic hypertension, on antihypertensive therapy, eclampsia, diabetes, autoimmune disease and renal disease were excluded.
Study population
One hundred and twenty-three (123) participants (≥ 20 weeks gestation) who met the eligibility criteria were recruited for this study. This comprised 53 age-matched, normotensive pregnant women, and 70 pregnant women with preeclampsia, receiving antenatal care at the O and G department of KATH. Participants with elevated blood pressure (≥ 140/90 mmHg) on two occasions, at least four hours apart, with visible dipstick proteinuria (≥ “+”), were considered to have PE.
Anthropometric measurements
Height (to the nearest 0.1 cm) and weight (to the nearest 0.1kg) were measured a wall-mounted ruler a bathroom scale (Zhongshan Camry Electronic Co. Ltd, Guangdong, China) respectively. Body mass index (BMI) was calculated using the formula; weight (kg)/height (m2).
Waist circumference (in centimetres) was measured with a Gulick II spring-loaded measuring tape (Gay Mill, WI). Hip circumference (in centimetres) was also measured in and the waist to hip ratio (WHR) was calculated by dividing the waist circumference (cm) by the hip circumference (cm).
Blood pressure measurement
Trained personnel took duplicate measurements of blood pressure with a mercury sphygmomanometer and stethoscope in accordance with the recommendation of the American Heart Association [9].
Urine macroprotein and microalbumin determination
Dipstick, semi-qualitative method was used to determine urine protein as per manufacturer’s instructions (CYBOW™ DFI Co Ltd, Gimhae-City, and Republic of Korea). The presence of urine protein in concentrations ≥ “+”, using the semiquantitative colour scale on the urine reagent dipstick was considered as proteinuria in participants with pre-eclampsia [ref]. Urine microalbumin was estimated based on the pyrogallol red molybdate complex method (ref).
Blood sample collection and biochemical analysis
After an overnight fast (8-12 hours), 6 ml of venous blood sample was taken from each participant into serum separator tubes, allowed to clot and then centrifuged at 500 g for 15 min. The serum was stored at - 80°C until assayed.
The remaining 2 ml were dispensed into tubes containing 2.5 μg K2EDTA. Platelet concentration (PLT) was determined by an automated blood analyzer CELL-DYN 1700®, version 1.08, (Abbott Diagnostics, Abbott Park, Illinois, USA) while serum Aspartate Aminotransferase (AST), Alanine Aminotransferase (ALT), Alkaline Phosphatase (ALK PHOS), albumin (ALB), serum creatinine (Scr) and uric acid were estimated on the ATAC 8000 Random Access Chemistry System (Elan Diagnostics, Smithfield, RI, USA).
Statistical analysis
Continuous variables were expressed as their mean ± SEM, while categorical variables were expressed as proportion. Comparisons of the women with PE against the control group were performed using unpaired t tests, Chi-square (χ2) tests, or Fisher exact tests where appropriate. GraphPad Prism version 5.00 for windows was used for these statistical analyses (GraphPad software, San Diego California USA, www.graphpad.com).
Also, association between spot urine protein and microalbumin and between uric acid and spot urine protein was by Pearson Correlation Coefficient. Sensitivity and specificity of microalbuminuria and uric acid as diagnostic markers was performed using receiver operating characteristics (ROC) curve. P < 0.05 was interpreted as statistically significant for all comparison.
Results
Of the 123 study participants studied, 53 (40%) were classified as controls and 70 (60%) as cases presenting with preeclampsia.
Table 1 shows the demographic, clinical and biochemical characteristics of the study participants. The preeclamptics had higher blood pressure (SBP and DBP) compared to the controls (p < 0.001). Also, biochemical parameters [(uric acid and hepatic enzymes (ALT and AST)] were significantly elevated in cases than the controls. The mean urine microalbumin concentration was higher in the preeclamptics than the controls (p=0.005) while the mean platelet count and serum albumin levels were similar in the cases and the controls (p=0.307).
| Parameters | Pre-eclampsia | Controls | P-value |
|---|---|---|---|
| N=70 | N=53 | ||
| Age (yrs) | 30.51 ± 0.75 | 30.52 ± 0.65 | 0.996 |
| BMI (kg/m2) | 25.09 ± 0.48 | 25.98 ± 0.68 | 0.276 |
| SBP (mmHg) | 161.60 ± 2.22 | 107.20 ± 1.36 | 0.001 |
| DBP (mmHg) | 101.90 ± 2.83 | 66.63 ± 1.33 | 0.001 |
| UA (umol/L) | 360.70 ± 18.28 | 165.80 ± 7.38 | 0.001 |
| ALB (g/l) | 32.50 ± 0.58 | 34.24 ± 0.69 | 0.058 |
| CRT (umol/L) | 73.07 ± 4.94 | 82.70 ± 2.88 | 0.128 |
| AST (IU) | 42.10 ± 5.42 | 22.39 ± 1.03 | 0.003 |
| ALT (IU) | 51.65 ± 12.74 | 9.316 ± 0.56 | 0.005 |
| PLT (106 ) | 242.20 ± 9.90 | 264.90 ± 22.04 | 0.307 |
| MA (mg/g) | 154.70 ± 14.75 | 85.60 ± 8.60 | 0.001 |
BMI=Body Mass Index, SBP=Systolic Blood Pressure, DBP= Diastolic Blood Pressure, ALB=Albumin, CRT=Creatinine, UA=Uric Acid AST=Aspartate Aminotransferase, ALT=Alanine Aminotransferase, PLT= Platelets, MA=Microalbumin
Table 1: Demographic, clinical and biochemical data of studied participants.
Table 2 shows the demographic, clinical and biochemical characteristics of study participants stratified by urine microalbumin concentration. The preeclamptics with microalbuminuria >30-300 mg/g had insignificantly elevated DBP (p=0.817), uric acid (p=0.588) Creatinine (p=0.331) and transaminases (p=0.760; p=0.832). However, platelets count (p=0.683) and serum albumin (p=0.611), SBP (p=0.856) were reduced in the preeclamptics with urine microalbumin levels <30 mg/g though the differences were not significant.
| Parameters | MA <30 (mg/g) | MA 30-300 (mg/g) | P-value |
|---|---|---|---|
| Age (yrs) | 31.50 ± 2.36 | 31.18 ± 0.82 | 0.919 |
| BMI (kg/m2) | 25.68 ± 1.53 | 25.06 ± 0.45 | 0.458 |
| SBP (mmHg) | 162.5 ± 7.50 | 160.7 ± 2.55 | 0.856 |
| DBP (mmHg) | 105.0 ± 2.89 | 106.0 ± 3.00 | 0.817 |
| UA (umol/L) | 317.5 ± 72.46 | 357.8 ± 18.94 | 0.588 |
| ALB (g/l) | 33.85 ± 1.99 | 32.49 ± 0.69 | 0.611 |
| CRT (µmol/l) | 55.20 ± 5.31 | 77.18 ± 5.88 | 0.331 |
| AST (U/L) | 36.35 ± 11.60 | 44.06 ± 6.57 | 0.76 |
| ALT (U/L) | 68.00 ± 1.155 | 55.41 ± 15.56 | 0.832 |
| PLT (106/L) | 251.50 ± 28.60 | 236.4 ± 9.50 | 0.683 |
MA=Microalbumin
Table 2: Demographic, clinical and biochemical data of studied participants stratified by concentration of urine microalbumin.
Table 3 represents the demographic, clinical and biochemical characteristics of studied participants stratified by serum uric acid concentrations. Preeclamptics with uric acid levels <360 umol/L had significantly elevated serum albumin levels compared to those with uric acid levels >360 umol/L but blood pressure [SBP (p=0.542) and DBP (p=0.512)] were reduced in preeclamptics with uric acid <360 umol/L. Conversely, serum creatinine (p=0.780), transaminases [AST (p=0.538) and ALT (p=0.051)], platelets (p=0.116) and urine microalbumin (p= 0.073) levels were elevated in the preeclamptics with >360 umol/L though not significant.
| Parameters | UA< 360 umol/l | UA > 360 umol/l | P-value |
|---|---|---|---|
| Age (yrs) | 30.50 ± 0.94 | 30.55 ± 1.30 | 0.978 |
| BMI (kg/m2) | 25.08 ± 0.61 | 25.11 ± 0.73 | 0.981 |
| SBP (mmHg) | 162.50 ± 2.64 | 159.50 ± 4.18 | 0.541 |
| DBP (mmHg) | 103.20 ± 3.40 | 99.18 ± 5.14 | 0.512 |
| ALB (g/l) | 33.33 ± 0.79 | 30.69 ± 0.56 | 0.036 |
| CRT (umol/l) | 72.13 ± 6.41 | 75.13 ± 7.35 | 0.780 |
| AST (U/L) | 39.81 ± 4.14 | 47.08 ± 14.89 | 0.538 |
| ALT (U/L) | 34.88 ± 8.15 | 88.24 ± 35.79 | 0.051 |
| PLT (106/l) | 231.70 ± 10.70 | 265.20 ± 20.68 | 0.116 |
| MA (mg/g) | 136.80 ± 14.68 | 193.60 ± 33.42 | 0.074 |
UA=Uric Acid
Table 3: Demographic, clinical and biochemical data of studied participants stratified by serum uric acid concentration.
Table 4 shows the demographic, clinical and biochemical characteristics of preeclamptic participants stratified by spot urine albumin concentration. The preeclamptic participants with spot urine albumin concentration ≥2+ were younger (p=0.010) and had higher urine microalbumin levels (p=0.024) than those with spot urine albumin <2+. Transaminases (ALT, AST), creatinine, albumin, blood pressure (DBP and SBP), uric acid and platelets were elevated in the preeclamptic with spot urine albumin ≥2+ though not significant (p>0.05).
| Parameters | Spot urine Albumin < 2+ dipstick |
Spot urine Albumin ≥2+ dipstick |
P-value |
|---|---|---|---|
| Age (yrs) | 34.20 ± 1.45 | 29.51 ± 0.84 | 0.012 |
| BMI (kg/m2) | 24.36 ± 0.90 | 25.29 ± 0.56 | 0.431 |
| SBP (mmHg) | 158.70 ± 5.42 | 162.40 ± 2.46 | 0.500 |
| DBP (mmHg) | 100.10 ± 7.19 | 102.40 ± 3.05 | 0.741 |
| UA (umol/l) | 355.30 ± 48.79 | 362.10 ± 19.36 | 0.880 |
| ALB (g/l) | 32.29 ± 1.06 | 32.56 ± 0.69 | 0.852 |
| CRT (umol/l) | 59.97 ± 11.76 | 76.64 ± 5.37 | 0.168 |
| AST (IU) | 33.31 ± 5.63 | 44.50 ± 6.71 | 0.401 |
| ALT (IU) | 29.30 ± 6.37 | 57.75 ± 16.06 | 0.363 |
| PLT (106/l) | 238.00 ± 21.20 | 243.30 ± 11.28 | 0.826 |
| MA (mg/g) | 91.31 ± 32.73 | 172.00 ± 15.88 | 0.024 |
Table 4: Demographic, clinical and biochemical data of studied participants stratified by spot urine albumin concentration.
Tables 5 and 6 show the demographic, clinical and biochemical characteristics of preeclamptics stratified by gestational age (in trimester). The preeclamptics in their second and third trimester had significantly elevated blood pressure (SBP and DBP), uric acid, hepatic transaminases compared to the controls. However, though platelet count, albumin, microalbumin and creatinine were elevated in the controls compared to the preeclamptics the difference was not statistically significant.
| Parameters | Control | Case | p-value |
|---|---|---|---|
| T2 | T2 | ||
| Age (yrs) | 31.21 ± 0.90 | 30.63 ± 1.28 | 0.722 |
| BMI (kg/m2) | 24.24 ± 0.91 | 23.54 ± 0.58 | 0.651 |
| SBP (mmHg) | 105.50 ± 2.28 | 168.80 ± 5.15 | < 0.001 |
| DBP (mmHg) | 65.71 ± 2.13 | 107.50 ± 5.26 | < 0.001 |
| UA (umol/L) | 163.60 ± 9.37 | 323.80 ± 26.83 | < 0.001 |
| ALB (g/l) | 35.25 ± 0.93 | 33.13 ± 2.34 | 0.317 |
| CRT (umol/l) | 82.85 ± 4.60 | 69.19 ± 6.01 | 0.105 |
| AST (IU) | 23.31 ± 1.64 | 24.10 ± 2.75 | 0.799 |
| ALT (IU) | 9.92 ± 0.92 | 14.76 ± 1.09 | 0.006 |
| PLT (106/L) | 247.20 ± 34.06 | 235.60 ± 24.05 | 0.835 |
| MA (mg/g) | 68.73 ± 13.74 | 167.60 ± 44.10 | <0.001 |
| CRT (umol/l) | 82.85 ± 4.60 | 69.19 ± 6.01 | 0.105 |
| AST (IU) | 23.31 ± 1.64 | 24.10 ± 2.75 | 0.799 |
| ALT (IU) | 9.92 ± 0.92 | 14.76 ± 1.09 | 0.006 |
| PLT (106/L) | 247.20 ± 34.06 | 235.60 ± 24.05 | 0.835 |
| MA (mg/g) | 68.73 ± 13.74 | 167.60 ± 44.10 | <0.001 |
T2= second trimester
Table 5: Demographic, clinical and biochemical data of studied participants stratified by gestational age (second trimester T2).
| Parameters | Controls | Cases | P-value |
|---|---|---|---|
| T3 | T3 | ||
| Age (yrs) | 28.46 ± 0.83 | 30.50 ± 0.84 | 0.164 |
| SBP (mmHg) | 108.30 ± 1.96 | 160.60 ± 2.41 | < 0.001 |
| DBP (mmHg) | 67.71 ± 2.17 | 106.50 ± 1.79 | < 0.001 |
| UA (umol/L) | 174.10 ± 12.33 | 365.40 ± 20.31 | < 0.001 |
| ALB (g/l) | 34.01 ± 1.02 | 32.42 ± 0.60 | 0.175 |
| CRT (umol/l) | 83.11 ± 2.69 | 73.57 ± 5.53 | 0.306 |
| AST (IU) | 20.47 ± 1.12 | 44.42 ± 6.05 | 0.019 |
| ALT (IU) | 9.52 ± 0.79 | 56.41 ± 14.30 | 0.049 |
| PLT (106/L) | 295.70 ± 39.42 | 243.00 ± 10.80 | 0.08 |
| MA (mg/g) | 82.19 ± 11.08 | 172 ± 9.50 | < 0.001 |
T3=Third Trimester
Table 6: Demographic, clinical and biochemical data of studied participants stratified by gestational age (third trimester).
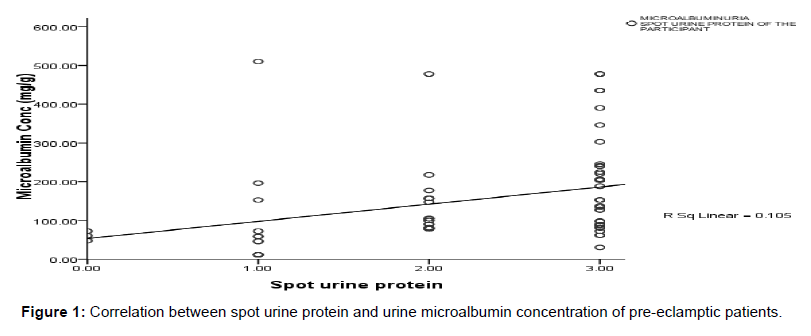
Figure 1 shows the relationship between spot urine protein and urine microalbumin. A significant positive linear correlation was observed between spot urine protein and urine microalbumin (r=0.324, p=0.006).
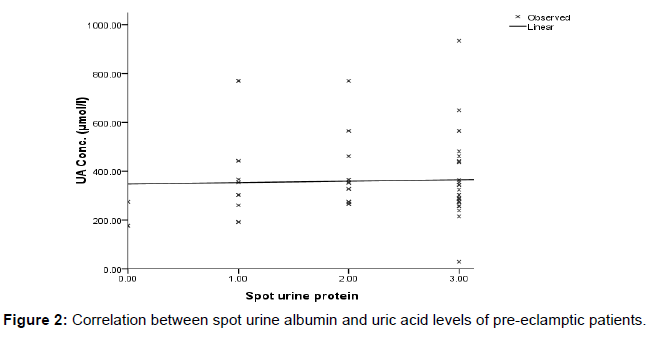
Figure 2 shows the relationship between uric acid and spot urine protein. A negative linear correlation was observed between uric acid and spot urine microalbumin (r=0.033, p=0.786).
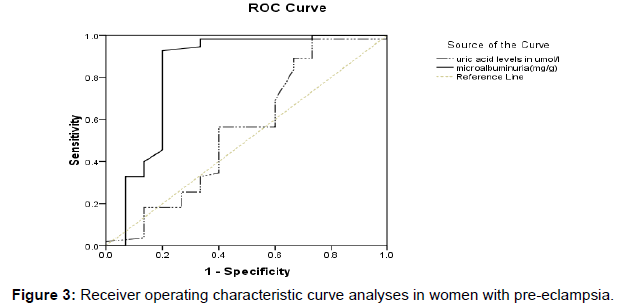
Figure 3 shows the Receiver operating characteristic curve analyses in women with preeclampsia. Urinary micro albumin value of 75.45 mg/g was identified as the best threshold to detect a spot urine protein of > +2 with a sensitivity of 92.7% and a specificity of 80.0%, PPV of 81.03% and NPV of 33.3%. Area under the curve = 0.835; asymptotic p-value of 0.000 at 95% CI (0.678-0.991). In contrast, serum uric acid level of 263.5 mg/g was identified as the best cut-off point to detect a spot urine protein of > +2 with sensitivity and specificity of 89.1% and 33.3% respectively PPV of 77.2% and NPV of 20.8%). Area under the curve = 0.552; asymptotic p-value of 0.538 at 95% CI (0.364-0.740).
Discussion
This study evaluated the accuracy of urine microalbumin and serum uric acid as diagnostic parameters among preeclamptics at the Komfo Anokye Teaching Hospital (KATH). In this study, the mean BMI of the preeclamptics were lower compared to the controls though the difference was not significant (p=0.276). This observation is consistent with the findings of Fatema et al., [10] and Anderson et al. [11] Also, the mean serum uric acid levels in this study were significantly higher (p=0.001) in participants with preeclampsia than in normal pregnant women and was consistent with previous reports by Koike T, et al. and Many A, et al. [12,13] The hyperuricaemia recorded in preeclamptics in this study could be a protective response, capable of opposing harmful effects of free radical activity as described by Many A, et al. [13]
Again, the preeclamptic patients excreted higher amount of urinary protein (p= 0.003) compared to healthy controls. In the present study microalbuminuria concentration for preeclamptics was markedly elevated, consistent with previous researches conducted by Poon LC et al and Rodriguez MH et al. [14,15] Moreover the current study recorded a high urinary protein with decreased serum albumin concentration among preeclamptic women compared to the healthy pregnant women though the difference was not statistically significant (p=0.0585). This finding is in accordance with reports of several studies [16,17] which concluded that preeclamptic patients have lower serum albumin concentration than normotensive controls. Furthermore, the findings of this study showed that the mean SBP and DBP were low among preeclamptics with uric acid levels >360 μmol/l compared with the group who had <360 μmol/l. According to Table 4, microalbuminuria concentration was significantly higher among preeclamptics in the ≥2+ spot urine albumin group than in <2+ spot urine albumin group. Also, the ≥2+ spot urine albumin group was significantly (p=0.0102) older compared to the other group. This explains age as a risk factor for preeclamptics who diagnostically present ≥2+ urine protein. In addition, there were no significant correlation between spot urine protein and uric acid levels (Figure 2) although the data in this study suggest a statistically significant difference between the mean uric acid value of the preeclamptics and healthy pregnant control groups (Table 2), its diagnostic ability for preeclampsia is questionable. Our study could not identify an obvious cut off point on the receiver operator curve (ROC) for uric acid level that was sufficiently sensitive and specific to distinguish preeclampsia. The best sensitivity (89.1%) and specificity (66.7%) was related to cut off point of 263.50 μmol/l. These figures are close to the values of some other studies, and the findings are consistent with those studies that did not find a clinical utility for serum uric acid in the prediction of preeclampsia [18,19] and in contrast to a study conducted by Roberts et al. [20] This might be because most of the studies that have reported a strong correlation between elevated serum uric acid and the severity of preeclampsia, have examined pregnant women with the most severe form of the disease. [21] This shows that a single estimate of uric acid is of little or no value in the prediction of preeclampsia. This is further confirmed by the insignificant (p=0.538) results shown by our ROC analysis (Figure 3).
Finally, a significant positive correlation (r=0.324, p=0.006) was established between spot urine protein (macroalbumin) and microalbuminuria. This finding partly agrees with the observations made in other studies where 24 hour urine was used in the detection of macroalbuminuria. [22,23] The use of microalbumin in the diagnosis will aid early detection of proteinuria a key diagnostic determinant of preeclampsia. The study is limited in the sense that it could not generalize the prevalence and incidence to the whole Ghanaian population in Kumasi, since it was a hospital-based study.
Conclusion
According to the ROC curve analysis, sensitivity (92.7%) and specificity (80.0%) of microalbuminuria were best identified at a threshold point of 75.45 mg/g with PPV of 81.03% and NPV of 33.3%. In comparison with uric acid accuracy, urinary microalbumin can be used as an alternative diagnostic marker to spot urine protein for predicting early onset of preeclampsia.
Acknowledgements
The authors are grateful to the patients and staff of the OG department of KATH and the staff of the laboratory unit of the Effiduase Government hospital for their immense contributions to this study.
Conflict of Interest
All authors disclose that there was no conflict of interest.
Availability of Data and Materials
Data will not be shared in order to protect the anonymity of the participants
Author Contribution
ME did the literature searches, and the bench work under the guidance and supervision of KB-A and CAT. ME and RKDE did the first draft of the manuscript. RKDE and EOA did the statistical analysis. KB-A and RKDE designed the experiments and did the final draft of the manuscript. CAT recruited and did the monitoring of the patients. All the authors read and approved the final manuscript.
REFERENCES
- ACOG: Diagnosis and management of preeclampsia and eclampsia. In: 2002.
- Sibai B. Hypertension. In: Obstetrics: Normal and Problem Pregnancies. Gabbe SG, Simpson JL, (Editors) Philadelphia: Saunders Elsevier; 2012.
- Fadel HE, Northrop G, Misenhimer HR. Hyperuricemia in pre-eclampsia. A reappraisal. Am J Obstet Gynecol 1976;125:640-647.
- Liedholm H, Montan S, Aberg A. Risk grouping of 113 patients with hypertensive disorders during pregnancy, with respect to serum urate, proteinuria and time of onset of hypertension. Acta Obstet Gynecol Scand Suppl 1984;118:43-48.
- Fadel HE, Sabour MS, Mahran M, Seif-el DD, el-Mahallawi MN. Serum uric acid in pre-eclampsia and eclampsia. J Egypt Med Assoc 1969;52:12-23.
- Lam C, Lim KH, Kang DH, Karumanchi SA. Uric acid and preeclampsia. Semin Nephrol 2005; 25:56-60.
- Siemons JM, Bogert LJF. The uric acid content of maternal and fetal blood. J Biol Chem 1917;32:63-67.
- Cote AM, Brown MA, Lam E, Von Dadelszen P, Firoz T, Liston RM, et al. Diagnostic accuracy of urinary spot protein: Creatinine ratio for proteinuria in hypertensive pregnant women: systematic review. BMJ 2008;336:1003-1006.
- KioscHos JM, Kirkendall WM, Valenca MR, Fitz AE. Unilateral renal hemodynamics and characteristics of dye-dilution curves in patients with essential hypertension and renal disease. Circulation 1967;35:229-249.
- Fatema K, Khatun M, Akter S, Ali L. Role of urinary albumin in the prediction of preeclampsia. Faridpur Med Coll J 2011;6:14-18.
- Anderson NH, McCowan LM, Fyfe EM, Chan EH, Taylor RS, Stewart AW, et al. The impact of maternal body mass index on the phenotype of pre-eclampsia: a prospective cohort study. BJOG, 2012;119:589-595.
- Koike T, Minakami H, Takayama T, Ogawa S, Kuwata T, Sato I. Elevation of the serum uric acid level preceding the clinical manifestation of preeclampsia in twin pregnancies. Gynecol Obstet Invest 1997;44:97-101.
- Many A, Hubel CA, Roberts JM. Hyperuricemia and xanthine oxidase in preeclampsia, revisited. Am J Obstet Gynecol 1996;174:288-291.
- Poon LC, Kametas N, Bonino S, Vercellotti E, Nicolaides KH. Urine albumin concentration and albumin-to-creatinine ratio at 11(+0) to 13(+6) weeks in the prediction of pre-eclampsia. BJOG 2008;115:866-873.
- Rodriguez MH, Masaki DI, Mestman J, Kumar D, Rude R. Calcium/creatinine ratio and microalbuminuria in the prediction of preeclampsia. Am J Obstet Gynecol 1988;159:1452-1455.
- Salari MD, Eftekhari MD. The comparison of total and ionized serum calcium in preeclamptic pregnant women and the women with normal pregnancy. Journal of Rafsanjani University of Medical sciences and Health Services 2005;4:123-128.
- Gojnic M, Petkovic S, Papic M, Mostic T, Jeremic K, Vilendecic Z, et al. Plasma albumin level as an indicator of severity of preeclampsia. Clin Exp Obstet Gynecol 2004;31:209-210.
- Cnossen JS, De Ruyter-Hanhijarvi H, Van der Post JA, Mol BW, Khan KS, Ter Riet G. Accuracy of serum uric acid determination in predicting pre-eclampsia: a systematic review. Acta Obstet Gynecol Scand 2006;85:519-525.
- Taefi A, Jamal A, Delavari H. The role of serum uric acid in preeclampsia. Journal of Family and Reproductive Health 2008;2:159-162.
- Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, et al. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension 2005;46:1263-1269.
- Acien P, Lloret G, Lloret M. Perinatal morbidity and mortality in pregnancy hypertensive disorders: prognostic value of the clinical and laboratory findings. Int J Gynaecol Obstet 1990;32:229-235.
- Al RA, Baykal C, Karacay O, Geyik PO, Altun S, Dolen I. Random urine protein-creatinine ratio to predict proteinuria in new-onset mild hypertension in late pregnancy. Obstet Gynecol 2004;104:367-371.
- Durnwald C, Mercer B. A prospective comparison of total protein/creatinine ratio versus 24-hour urine protein in women with suspected preeclampsia. Am J Obstet Gynecol 2003;189:848-852.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.