Effect of Bleaching with Kiwi Extract and Hydrogen Peroxide on Color Change of Enamel Surface
Received: 10-Nov-2022, Manuscript No. AMHSR-23-85531; Editor assigned: 15-Nov-2022, Pre QC No. AMHSR-23-85531(PQ); Reviewed: 29-Nov-2022 QC No. AMHSR-23-85531; Revised: 02-Jan-2023, Manuscript No. AMHSR-23-85531(R); Published: 30-Jan-2023
Citation: Jackson K, et al. Effect of Bleaching with Kiwi Extract and Hydrogen Peroxide on Color Change of Enamel Surface. Ann Med Health Sci Res. 2023;13:1-5.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Introduction: The find and replace the chemical bleaching agent namely the Hydrogen Peroxide (HP) with a naturally found fruit or substance to whiten a discolored tooth is a need of the hour.
Aim: To evaluate and compare the color changes in enamel surface following bleaching with 35% hydrogen peroxide and 2% Kiwi Extract (KE).
Materials and Methods: In this in-vitro study, 15 maxillary anterior teeth were decoronated and allocated randomly to two groups; group 1 (negative control) (n=5) and group 2 (experimental) (n=5). To evaluate the bleaching efficacy, group 2 specimens were experimentally stained with tea. Baseline color coordinates (pre-bleach values) (L*, a*, b*) (ΔE) were recorded. The crowns were sectioned to obtain four equal parts to obtain 15 specimens and these were allotted randomly to 3 subgroups a, b, c (n=5 each) based on the bleaching protocol. Subgroup a, Hydrogen Peroxide (HP); subgroup b, Kiwi Extract (KE); subgroup c, HP+SE (HPS). Following bleaching, color coordinates (post-bleach values) (L*, a*, b*) (ΔE) were measured as mentioned before. Kruskal-Wallis one-way Analysis of Variance (ANOVA) was used to calculate the p-value and Post-Hoc Tukey Honest Significant Test (HSD) was used to identify the signi icant groups, (p-value<0.05).
Results: Post bleach values were higher for group C (kiwi extract+hydrogen peroxide), when compared to post bleach value means obtained from group A and B.
Conclusion: Kiwi extract alone or in combination with hydrogen peroxide is an efficient and effective dental bleaching agent.
Keywords
Antioxidant; Kiwi; Color; Tooth enamel
Introduction
The desire for whiter teeth among the general population has reached an all-time high with many individuals seeking professional help for non-invasive bleaching techniques. Despite various potential side-effects of peroxide, it has remained the active ingredient in tooth whitening agents in the form of Hydrogen peroxide and carbamide peroxide [1]. There is evidence to show that 35% HP results in shallow erosions, sensitivity, structural alterations in enamel structure and compromised bonding of resin composite [2,3].
Attempts to minimize these adverse effects by incorporating remineralising agents, calcium and fluoride substitutes, the results have met with limited success [4]. Furthermore, compromised bonding of adhesive restorations to bleached teeth is an important clinical concern. The use of antioxidants and proanthocyanidins appear to completely neutralize the deleterious effects of bleaching and increase the micro-shear bond strength significantly [5-8]. However, it is important to note that these adjunct treatments were unable to reverse the structural damage of the enamel surface caused by the bleaching agent [9].
Recently there has been an upsurge in usage of natural substances for treatment of various diseases as they minimize or prevent the side effects [10,11]. Similarly, this has led to a constant search for replacing the existing chemicals in cosmetic dentistry used for various purposes. This change would be welcomed in dentistry if there is an alternative for hydrogen peroxide due to its various adverse effects on the enamel surface.
Materials and Methods
Study setting
This in-vitro study was conducted in the department of aesthetic dentistry, Saveetha institute of medical and technical sciences, Chennai, Tamil Nadu, India. Study protocol was approved by the institutional review board and ethical committee of the university.
Preparation of extract solution for study
The fresh pulpy kiwi was bought 200 gm of kiwi fruits were cleaned, cut into round shapes and blended with 15 mL of distilled water in a blender to obtain about 50 mL of kiwi concentrate. This concentrate was filtered and then transferred into a cooling centrifuge to be processed at 2000 rpm for about 20 minutes at a temperature of 40°C. The liquid thus collected (2% kiwi extract) was stored at 4°C till further use (Figures 1-4).
Specimen collection
Human natural teeth were used in this study. Single rooted, caries free, maxillary anterior teeth (Central, lateral incisors and canines) (n=15) were taken from oral biology department, Saveetha Dental college. Teeth with fractures, cracks and stains were excluded. The experiments were performed in accordance with the appropriate guidelines [12]. The collected teeth were decoronated and were stored in 0.2% thymol and kept hydrated until use (Figures 5 and 6).
Staining of teeth and bleaching protocol
The teeth were sectioned at the cemento-enamel junction so as to obtain the crown portion and were stored in saline until it was subjected to further experimental procedures. A number of 5 teeth served as negative control (group A) and did not receive any bleaching procedure nor staining.
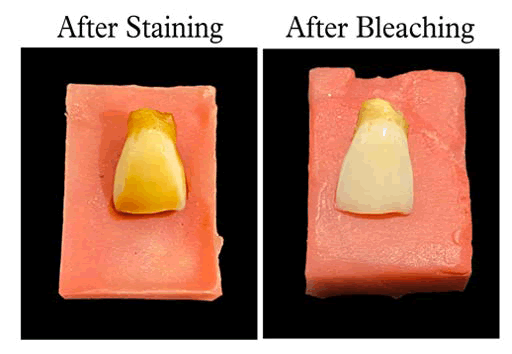
The remaining 5 were experimental specimens (group B) were stained artificially based on a previously established protocol as described by Sulieman M. A 2 g tea bag was suspended in boiling deionized water (100 mL) for 5 minutes and then it was cooled down to room temperature. The clear solution followed by straining the solution was used for staining the teeth. Clear tea solution was obtained by straining the solution. The specimens were then immersed in the tea solution for 24 hours, after which they were removed and subjected to reflectance spectrometer to record the baseline color variables (pre-bleach) (L*, a*, b*-ΔE values). The crowns were then sectioned longitudinally and horizontally to obtain four equal parts (15 specimens); each part being randomly allocated to one sub-group (a,b,c n=5 each), depending on the bleaching protocol as stated below [12]. The specimens were mounted on self-curing acrylic resin blocks, exposing only the labial surface. Group A (n=5): Negative control (N); Group B (n=5): 35% HP; Group C (n=5): HP +KE (1:1).
The labial surface of the specimens of group B and C was exposed to the respective bleaching agents for 5 minutes using an applicator tip replenishing every minute. The teeth were then rinsed and stored in artificial saliva at room temperature for 24 hours. All the specimens were further subjected to spectrometer to obtain the post-bleach color variables L*, a*, b* according to the CIE L*a*b color system. The color change (ΔE) was calculated using the formula [13]:
ΔE={(ΔL*) 2+(Δa*) 2+(Δb*) 2}1⁄2, Where L*, a*, b* represents the value of color using hue, value and chroma. To evaluate the whiteness after bleaching, the color changes before and after bleaching was taken as an index. All the procedures were performed at room temperature by the same operator under aseptic conditions.
Statistical analysis
Kruskal-Wallis one-way ANOVA and Post-hoc Tukey’s test were used to test the statistical significance. The p-value was set at 5%.
Results and Discussion
Hydrogen Peroxide is the most commonly used dental bleaching agent. Ionic dissociation of hydrogen peroxide releases free radicals namely nascent oxygen, per-hydroxyl, hydroxyl radical and superoxide anions [14]. These highly reactive radicals disrupt the electron conjugation by breaking down the highly conjugated organic molecules involving carbon, nitrogen and oxygen atoms, into smaller, less pigmented ones, thus changing the absorption energy of the molecule. The consequent shift of the visible absorption spectrum of the compound from a longer to a shorter wavelength (a colourless compound) forms the basis of whitening/ bleaching action on the substrate.
With regards to the bleaching effectiveness, pre-bleaching values of all the groups were not significant, implying equal distribution of specimens within the groups. Post-bleaching delta E values of all the groups were statistically significantly higher compared to the pre-bleach values (p<0.05). Higher the delta E values, better is the stain removal hence more whiter is the surface of the teeth. Thus, post-bleach specimens were whiter in color compared to pre-bleach specimens. This suggests that all treatments bleached effectively the teastained enamel (more whiter). All treatment groups (postbleach), showed statistically significant differences in the ΔE values (p<0.05). When comparing the bleaching agents alone, the teeth bleached with kiwi extract were as white as compared to hydrogen peroxide, i.e., it was not statistically significant (p>0.05), suggesting that kiwi extract has equal bleaching efficacy as the standard hydrogen peroxide.
Hydrogen peroxide (35%) appears to have a concentrationdependent bleaching effectiveness, which is attributed to higher content of the low molecular weight oxygen free radicals (34.014 g/mol). The similar efficacy of kiwi extract as compared to Hydrogen Peroxide may be attributed to its pH (3.27-3.86) and the presence of the acidic components namely ascorbic acid. Ascorbic acid in kiwi may increase the surface energy by removing the surface debris, thereby allowing the citric and maleic acid to penetrate into the tooth surface to remove the surface stains. This may also explain the synergistic color change in the combination group (C).
Both peroxide and the acids could have caused disruption of more complex stain molecules into smaller simpler molecules making it colorless (Tables 1-3 and Figure 7).
| Table 1: Descriptive statistics comparing the mean and standard deviation of pre-bleach ∆E values for all the 3 group specimens. | ||||||
| Groups | N | Mean | Std. dev | Std. error | Minimum | Maximum |
|---|---|---|---|---|---|---|
| A (HP) | 5 | 11.59 | 0.33 | 0.08 | 11.09 | 12.12 |
| B (KE) | 5 | 11.55 | 0.43 | 0.11 | 11 | 12.17 |
| C (HP+KE) | 5 | 11.59 | 0.22 | 0.03 | 11.3 | 12.21 |
Note: HP: Hydrogen Peroxide; KE: Kiwi extract; HP+KE: Hydrogen Peroxide and Kiwi Extract
| Table 2: Descriptive statistics comparing the mean and standard deviation of post-bleach ∆E values for all 3 group specimens. | ||||||
| Groups | N | Mean | Std. dev | Std. error | Minimum | Maximum |
|---|---|---|---|---|---|---|
| A (HP) | 5 | 19.1 | 0.66 | 0.15 | 17.09 | 17.12 |
| B (KE) | 5 | 18.34 | 0.42 | 0.1 | 18.05 | 18.22 |
| C (HP+KE) | 5 | 22.73 | 2.12 | 1 | 19.02 | 29.21 |
| Table 3: Comparison of the mean and standard deviation of pre and post- bleach ∆E values for all the group specimens. p-value (<0.05). | ||
| Groups | Pre-bleach (∆E values) | Post-bleach (∆E values) |
|---|---|---|
| Group A | 11.59 ± 0.33 | 19.10 ± 0.66 |
| Group B | 11.55 ± 0.43 | 18.34 ± 0.42 |
| Group C | 11.59 ± 0.22 | 22.73 ± 2.12 |
Conclusion
This study highlights that enamel bleaching with kiwi extract, effectively bleaches the surface, without significant adverse effects on the enamel surface. However, it remains unknown if kiwi extract, alone or in combination with hydrogen peroxide can achieve the same bleaching effect as hydrogen peroxide alone, without the disadvantages of hydrogen peroxide such as surface erosion and compromised bonding of resins. Hence, this study was designed to comparatively investigate the effect of kiwi extract, with and without hydrogen peroxide on the bleaching effectiveness. The null hypothesis was that kiwi extract does not significantly improve bleaching, compared to hydrogen peroxide alone.
Acknowledgment
We would like to acknowledge the Faculty, Students and staff of the department of aesthetic dentistry. The IT department and the management of Saveetha dental college and hospitals for their support and making the patient details and records available for carrying out this study.
Conflict of Interest
The authors declare no conflict of interest.
Source of Funding
The present study was supported by the following agencies
• Saveetha dental college
• Saveetha institute of medical and technical science
• Saveetha university
References
- Carey CM. Tooth whitening: What we now know. J Evidence-Based Dent Practice. 2014;14:70-76.
[Crossref] [Googlescholar] [Indexed]
- Xu B, Li Q, Wang Y. Effects of pH values of hydrogen peroxide bleaching agents on enamel surface properties. Operative Dentistry. 2011;36:554-562.
[Crossref] [Googlescholar] [Indexed]
- Sulieman M, Addy M, Macdonald E, Rees JS. A safety study in vitro for the effects of an in-office bleaching system on the integrity of enamel and dentine. J Dentistry. 2004;7:581-590.
[Crossref] [Googlescholar] [Indexed]
- Borges AB, Samezima LY, Fonseca LP, Yui KC, Borges AL, Torres CR. Influence of potentially remineralizing agents on bleached enamel microhardness. Operative Dentistry. 2009;34:593-597.
[Crossref] [Googlescholar] [Indexed]
- Lai SC, Tay FR, Cheung GS, Mak YF, Carvalho RM, Wei SH. Reversal of compromised bonding in bleached enamel. J Dental Res. 2002;81:477-481.
[Crossref] [Googlescholar] [Indexed]
- Türkün M, Kaya AD. Effect of 10% sodium ascorbate on the shear bond strength of composite resin to bleached bovine enamel. J Oral Rehabilitation. 2004;31:1184-1191.
[Crossref] [Googlescholar] [Indexed]
- García-Godoy F, Dodge WW, Donohue M, O'Quinn JA. Composite resin bond strength after enamel bleaching. Operative Dentistry. 1993;18:144-147.
[Googlescholar] [Indexed]
- Kimyai S, Oskoee SS, Rafighi A, Valizadeh H, Ajami AA, Helali ZN. Comparison of the effect of hydrogel and solution forms of sodium ascorbate on orthodontic bracket-enamel shear bond strength immediately after bleaching: An in vitro study. Ind J Dental Res. 2010;21:54-58.
[Crossref] [Googlescholar] [Indexed]
- Kalliath C, Mukunda A, Pynadath M, Venugopal V, Prethweeraj J. Comparison between the effect of commercially available chemical teeth whitening paste and teeth whitening paste containing ingredients of herbal origin on human enamel. Ayurveda. 2018;39:113-117.
[Crossref] [Googlescholar] [Indexed]
- Pramesti A, Jasrin TA, Hidayat OT. Teeth re-whitening effect of strawberry juice on coffee stained teeth. Padjadjaran J Dent. 2013;25.
[Googlescholar] [Indexed]
- Gopinath S, James V, Vidhya S, Karthikeyan K, Kavitha S, Mahalaxmi S. Effect of bleaching with two different concentrations of hydrogen peroxide containing sweet potato extract as an additive on human enamel: An in vitro spectrophotometric and scanning electron microscopy analysis. J Conservative Dent. 2013;16:45-49.
[Crossref] [Googlescholar] [Indexed]
- Chng HK, Ramli HN, Yap AU, Lim CT. Effect of hydrogen peroxide on intertubular dentine. J Dent. 2005;5:363-369.
[Crossref] [Googlescholar] [Indexed]
- Seghi RR, Denry I. Effects of external bleaching on indentation and abrasion characteristics of human enamel in vitro. J Dent Res. 1992;71:1340-1344.
[Crossref] [Googlescholar] [Indexed]
- Alqahtani MQ. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent J. 2014;2:33-46.
[Crossref] [Googlescholar] [Indexed]








 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.