Effects of Incorporation of Various Amounts of Zirconium Oxide Particles on Microstructure and Mechanical Strength of Conventional and Light-cure Glass Ionomer Cements
2 Department of Oral and Maxillofacial Surgery, School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
3 Faculty of Dentistry, Guilan University of Medical Sciences, Rasht, Iran
4 School of Dentistry, Isfahan University of Medical Sciences, Isfahan, Iran
Citation: Fazelian N, et al. Effects of Incorporation of Various Amounts of Zirconium Oxide Particles on Microstructure and Mechanical Strength of Conventional and Light-cure Glass Ionomer Cements. Ann Med Health Sci Res. 2018;8:365-369
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: The purpose of this study is to investigate the mean compressive strength and microstructure of the light-cure and self-cure glass ionomer by adding 5%, 10%, and 15% zirconium oxide particles. Methods: In this experimental study, 112 specimens in 8 groups including control groups (light-cure and self-cure glass ionomer) and 3 groups containing zirconium oxide particles (weight percentages 5%, 10% and 15%) in light-cure glass ionomer and 3 groups containing zirconium oxide particles (weight percentages 5%, 10% and 15%) placed in self-cure glass ionomer. The specimens were made in a transparent plastic mold with a diameter of 4 and a height of 6 millimeters. Lightcure glass ionomer cements were emitted from the light cure unit after 80 seconds light radiation. Then all specimens were stored in distilled water (37°C) for one day. Then they were subjected to compressive strength test using the Universal Testing Machine (STM- 20) (SANTAM) with 2 N initial force and 0.5 mm/min blade speed). The compressive strength (MPa) was analyzed by one- way ANOVA and HSD post hoc tukey (p<0.05). Results: In light-cure glass ionomer groups, the highest and lowest mean compressive strength were observed for groupe containing 15% w zirconium oxide particles (228.51) and control group (170.44). In the self-cure glass ionomer group, the highest and lowest mean compressive strength were observed for groupe containing 10% w zirconium oxide particles (36.04) and control group (30.58). Conclusion: Based on the results of this study, it can be concluded that the addition of zirconium oxide particles 15% w in light-cure glass ionomer increases the mean compressive strength.
Keywords
Glass ionomer; Mechanical strength; Microstructure; Zirconium oxide particles
Introduction
Glass ionomer is a material that has been introduced in dentistry since 1970 and has been used as one of the tooth-colored materials in restorative dentistry. [1] Other than the chemical bonding ability to the tooth, other advantages of this material such as the thermal expansion coefficient similar to the tooth, the thermal transfer coefficient similar to the tooth, the absence of contraction during the polymerization, unlike the composites caused that glass ionomer still being considered as one of the important restorative materials in dentistry. [2] Glass ionomer cements have been used in dentistry for about thirty years. The invention of the glass ionomer cement in 1969 by Wilson and Kent was the direct result of primary studies on silicate cements of dentistry. [3] Glass ionomer cements look like silicate cements and similar to polycarboxylate cements in terms of cohesion. The glass ionomer cement compounds are complex and diverse. Even two commercial samples are not identical in terms of composition and may also be different in terms of quality. However, some chemical properties are common in this material. The main material in the glass ionomer cement is calcium fluoroalumino silicate. [4] Three main components of the glassy ionomer cements used in dentistry are silica (SiO2), alumina (Al2O3) and calcium fluoride (CaF2) when they are bonded together; they create a suitable glass structure to form cements. In glass ionomer cements, the reaction of hardening and cement forming is based on the acid-base reaction. The hardening mechanism includes dissolving the glass particles and releasing aluminum and calcium ions, which are then combined with polyacrylic acid to form calcium and aluminum polyacrylate chains. Calcium and aluminum ions create a porous network by forming crosslink with polyacid chains that allow free flow of hydroxyl and fluoride ions into and out of the underlying material of cement. The reaction of hardening of the glass ionomer is a long-term reaction that lasts up to one month after the substitution of material. [5] In fact, it can be said that the glass ionomer is an unusual material with superior quality and different from other materials. Its semi-transparent property is similar to dental porcelain and it sticks to the tooth structure. [3] These materials bond to enamel and dentin permanently, [6] which causes the seam between the dental material and tissue almost completely closed and prevents the penetration of causes of decay and thus prevents the appearance of secondary decay. [2,7] These materials have fluoride release properties for a long time and are able to absorb fluoride when exposed to a fluoride solution, thereby inhibit the development of decay in adjacent tooth tissue. [3] In fact, it can be said the capability of releasing fluoride and the chemical real band and their micromechanical bond with the tooth are two main attributes which proposes choice of this cement in patients with multiple carious lesions and high risk, as well as repair of cervical lesions. [8]
Glass ionomer cement is used as a restorative material or under other fillings as an insulating and protective of tooth tissue. Due to the fact that this cement releases fluoride, dental decay is largely prevented. [9] From the general point of view, it can be argued that glass ionomers are commonly used to restore dental structures in clinical dentistry. Like other dental materials, the glass ionomer cements also have weaknesses, which mainly include sensitivity to moisture and low initial strength. Efforts have been done to improve the properties of initial glass ionomer cements and to remove the weaknesses mentioned above. These efforts include changes in the structure of the glass ionomer powder and the polyacrylic acid, which created as a result of obvious differences in the chemical composition and physical properties and the way of using different types of commercial materials. [10] One of the strategies used to increase the mechanical properties of glass ionomer cements is the use of metal fillers. Accordingly, for the first time, an alloy of silver was used in glass ionomer. [11]
Based on the curing process, the glass ionomers can be divided into two types of self-cure and light-cure that in self-cure, curing is occurred based on a spontaneous reaction in Light-cure exposed to visible light. Old ionomer cements have also been modified with the addition of light cure resin monomers and are referred to as resin-modified glass ionomer cements. [12,13] In this category of glass ionomers, the addition of resin improves mechanical properties. [14-16] The effect of the use of alumina and zirconia fillers as metal fillers has been investigated on the strength of light-cure glass ionomer, however, self-cure glass ionomers have not been studied in this regard, and in this study, we intend to study the effect of using zirconium oxide particles on the mechanical strength and microstructure of the self-cure and light-cure glass ionomer. In the study of Souza et al. [17] various percentages of zirconia (9.4%, 11% and 15.8%) were added to the light-cure glass ionomer and had a negative effect on the compressive strength of light-cure glass ionomer. In this study, the percentage of different fillers was added to self-cure and light-cure glass ionomers than the study of souza.
Materials and Methods
Synthesis of the glass ionomer cements
Different weight percentages including (0% - 5% - 10% - 15%) from zirconium oxide particles to the light-cure glass ionomer powder of Gold brand light-cured universal restorative from GC and self-cure glass ionomer powder of Gold brand universal restorative was added from GC and mixed by Mortar & Pestle method for 20 minutes to obtain a uniform distribution of particles. [18] A transparent plastic mold with a diameter of 4 and a height of 6 mm was chosen. The glass ionomer powder with different percentages of zirconium oxide particles was mixed with a specific ratio of liquid/powder to complete a complete transparent plastic mold in less than 25 seconds on a glass block. The experimental cement mixed was placed on the mold and was completely compressed in mold and covered with a glass slide. The light-cure glass ionomer groups were polymerized using LED device of Demetron (USA, kerr). Light-cure instrument with 8 mm diameter was so that all areas were exposed to radiation for 40 seconds. Then, we returned the specimen and exposed to radiation similarly. i.e., they were under light-cure for 80 seconds. All specimens were then stored in distilled water (37°C) for one day
The reinforced glass ionomer contains zirconium oxide particles and classified according to the percentage of zirconium oxide particles in eight groups:
• Light-cure glass ionomer
• Light-cure glass ionomer reinforced with zirconium oxide particles 5%
• Light-cure glass ionomer reinforced with zirconium oxide particles 10%
• Light-cure glass ionomer reinforced with zirconium oxide particles 15%
• Self-cure glass ionomer
• Self-cure glass ionomer reinforced with zirconium oxide particles 5%
• Self-cure glass ionomer reinforced with zirconium oxide particles 10%
• Self-cure glass ionomer reinforced with zirconium oxide particles 15%
The method of measuring the compressive strength
Then the compressive strength of specimen was obtained using the Universal Testing Machine Model STM-20 (SANTAM) with 2 N initial force and 0.5 mm/min blade speed by the following formula: [18,19]
CS=F/πr2
Microscopic analysis
A specimen from each group was used for investigating using SEM microscopy. Each specimen was attached to a stub with a doublesided carbon tape. Then place it inside the ION SPUTTERING for 2 minutes until the surface of the specimens is coated with gold and to be conductive. Finally, the specimen was placed into SEM devise JEOL brand JSM-840A model, and we photographed with Kev 15 voltage and the magnifications.
The results were described using appropriate statistical indices (mean, standard deviation) as well as appropriate statistical charts and using statistical models appropriate to the collected data were analyzed, such as one-way ANOVA or Kruskal-Wallis method, as well as appropriate Post Hoc tests, such as Tukey and Dunnett’s methods and independent t-test.
Results
Compressive strength
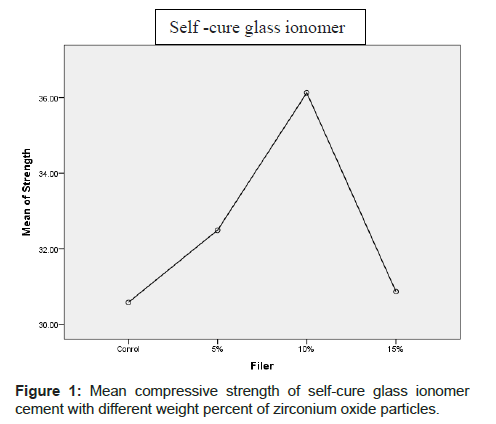
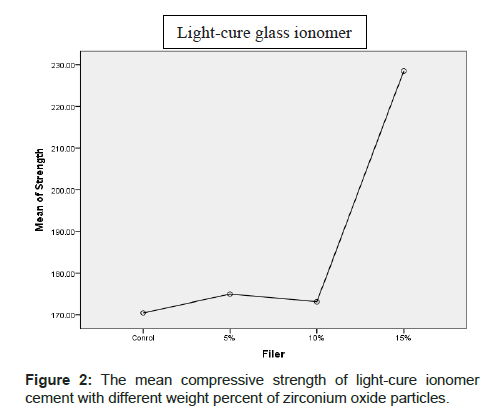
The mean compressive strength (MPa) and standard deviation of various cements are shown in Table 1 and Figures 1 and 2. In light cure Glass-ionomer groups, the highest and lowest mean compressive strength and standard deviation were observed for groupe containing 15% w zirconium oxide particles (228.51 ± 54.12) and control group (170.44 ± 16.27).
| Group | N | Mean | Std. Deviation | Minimum | Maximum | |
|---|---|---|---|---|---|---|
| Light-cure glass ionomer | Control | 14 | 170.4493 | 16.27260 | 146.17 | 196.17 |
| 5% | 14 | 174.9750 | 40.23328 | 106.36 | 263.37 | |
| 10% | 14 | 173.1343 | 31.45215 | 118.08 | 242.20 | |
| 15% | 14 | 228.5150 | 54.12520 | 129.29 | 318.66 | |
| Total | 56 | 186.7684 | 44.33482 | 106.36 | 318.66 | |
| Self-cure glass ionomer | Control | 14 | 30.5829 | 16.75803 | 14.84 | 81.21 |
| 5% | 14 | 32.4929 | 10.13337 | 18.94 | 49.68 | |
| 10% | 14 | 36.1293 | 17.04186 | 13.28 | 72.29 | |
| 15% | 14 | 30.8657 | 15.74351 | 15.15 | 71.33 | |
| Total | 56 | 32.5177 | 14.92809 | 13.28 | 81.21 | |
Table 1: Mean and standard deviation and max and min compressive strength of light-cure and self-cure glass ionomer cement with different percentages of zirconium oxide particles.
In the self-cure glass ionomer group, the highest and lowest mean compressive strength and standard deviation were observed for groupe containing 10% w zirconium oxide particles (36.04 ± 17.04) and control group (30.58 ± 16.75).
The results of Onway ANOVA analysis showed that statistically a significant difference was not observed in the mean compressive strength of self-cure glass ionomer containing 5%, 10% and 15% w zirconium oxide particles with normal control group (p>0.05). Also, statistically a significant difference was not observed in compressive strength of light-cure glass ionomer containing 5% and 10% w of zirconium oxide particles in control group. The mean compressive strength of light-cure glass ionomer containing 15% zirconium oxide particles had statistically a significant difference with control group (p<0.05).
Microstructure
SEM glass ionomer images show uniform distribution of Zirconia filers (A-H). Images A to D are related to self-cure glass ionomer. The image of A self-cure glass ionomer does not contain zirconium oxide particles. The images B to D are also related to the self-cure glass ionomer containing weight percentages 5%, 10%, and 15% of zirconium oxide, respectively, as seen by increasing Zirconium oxide particles, the amount of cracking increases. E-H-images are related to light-cure glass ionomer. The E image of self-cure glass ionomer does not contain zirconium oxide particles. Images F to H are also related to light cure glass ionomer containing weight percentages 5%, 10%, and 15% zirconium oxide particles, respectively, as seen by increasing percentage of Zirconium oxide particles, the size and amount of pores increases [Figure 3].
Figure 3: SEM images of glass ionomer cements with different weight percent of zirconium oxide particles. A: Self-cure glass ionomer cement (control group); B: Self-cure glass ionomer cement containing 5% weight of zirconium oxide particles; C: Self-cure glass ionomer cement containing 10% weight of zirconium oxide particles; D: Selfcure glass ionomer cement containing 15% weight of zirconium oxide particles; E: Light-cure glass ionomer cement (Control group); F:Light-cure glass ionomer cement containing 5% weight of zirconium oxide particles; G: Light-cure glass ionomer cement containing 10% weight of zirconium oxide particles; H: Light-cure glass ionomer cement containing 15% weight of Zirconium oxide particles.
Discussion
Resin-modified glass ionomer cements are used in patients with high risk of decay due to the release of fluoride and the chemical and micromechanical bond to the dental structure. [20] The glass ionomer has many uses as luting cement, filler and liner. However, its poor mechanical properties limit its applications in high stress areas. [21,22] Most of the chewing forces have a compressive nature. [23] Therefore, compressive strength testing is used to simulate the stress caused by the forces involved in the restoration, the base, the liner, or core build-up material. [24] However, there are disagreements over different mechanical tests to evaluate the mechanical properties of cements.
Dowling et al. suggested that compressive strength is not a suitable test for fragile materials such as glass ionomer, since the data obtained from this test is very diverse. [25] While Kleverlaan et al. stated that the mechanical integrity of material can be measured by compressive strength test, [26] a compressive strength test was used to simulate the chewing forces in this study.
In a study conducted by Strassler et al. in 2011 regarding the use of glass ionomers as a restoration, the results of the study showed that the compressive strength of light-cure glass ionomer is in the range of 184- 117 Mpa, [27] while for self-cure glass ionomer are reported in the range of 10-15 Mpa. [28] Several strategies have been proposed to improve the physical properties of the glass ionomer, including the addition of filler to the glass ionomer material. [22] In a study conducted by Arcis et al. in 2002 regarding the mechanical properties of light-cure resins hydroxyapatite with hydroxyapatite, the results of the study showed that the addition of nanohydroxyapatite to the glass ionomer enhances the mechanical properties of the material. [29]
In 2011, Elsaka et al., by adding nanoparticles of titanium oxide to the glass ionomer, concluded that titanium oxide nanoparticles increase mechanical strength and could be used in high stress areas such as class II, I restorations. [30]
Zirconium oxide particles due to strength and modulus and higher hardness increase the compressive strength significantly. [31] Therefore, in the present study, Zirconium oxide particles were added to increase the compressive strength of the glass ionomer to the light-cure and selfcure glass ionomer. The results showed that adding zirconium oxide particles up to 15% w to light-cure glass ionomer (fujiII LC) increased the mean compressive strength. However, the percentages of 5% and 10% of zirconium oxide particles had a mean compressive strength similar to control group of light-cure glass ionomer. On the other hand, the addition of weight percentages 5%, 10% and 15% zirconium oxide particles to self-cure glass ionomer did not show a significant difference in mean compressive strength.
In the study conducted by Souza et al. [17] adding different percentages of Zirconia (9.4%, 11% and 15.8%) to light-cure glass ionomer had a negative effect on the compressive strength of light-cure glass ionomer, which, in percentages of zirconia particles is according to the results of this study but in 15% weight is in contradiction with the results of our study, one of the reasons is that in our study zirconium oxide particles were used, but in the souza study, pure zirconium fillers were used, on the other hand, in the study of souza nanoparticles were used but in this study particles with micrometer dimensions were added to the powder, which large particle sizes can increase strength against pressure force.
In a study conducted by Gu et al. the mixture of nano-particles of hydroxyapatite and zirconium oxide with certain percentages was added to the conventional glass ionomer powder, and the volumetric percentages of 4% and 12% showed higher compressive and tensile strength than the initial cement. [31] But in this study, the addition of weight percentages 5%, 10%, and 15% w of zirconium oxide particles to self-cure glass ionomer sulfate did not have a significant difference in mean compressive strength, which could be related to the absence of hydroxyapatite particles because the above particles can easily be reacted with glass ionomer powder and create a uniform mixture.
SEM is a tool for showing surface morphology, filler size, and uniformity and porosity distribution. [32] The reason for the presence of porosity in cement without the addition of zirconium oxide particles is that during spatulation the air bubbles embeded in cement and then will appear as porosity.
When specimens were analyzed using SEM, a uniform, dense distribution of the glass ionomer and zirconium oxide particles was observed in the matrix, which was a key factor in improving the mechanical properties. However, the presence of cracks observed in the structure set was due to the weakness of ZrO2 glass interface compared to the control group (Light-cure and self-cure glass ionomer). Fleming et al. consider the reason for the presence of pores in micrographs of glass-ionomer samples mixed with the filler due to the trapping of air bubbles in the manipulation. [32]
Additionally, the water inside the glass ionomer is sensitive to environmental conditions such as drying and synersis during the initial phase of reaction. During the sting reaction, a small amount of water tightly attaches to the material structure during the reaction, while more unreacted water remains in the material structure that separates from the material structure during the synersis phenomenon. The presence of cracks and pores in the material structure is related to the contraction of polymerization of material during the sting reaction, which results in stress between the matrix and filler phase. During the drying steps of specimens for observing with SEM, the amount of water not reacted in the reaction are lost and causes the cracks and more pores in the material structure. [17]
In the micrographs of SEM, in the present study, in the samples of selfcure glass ionomer specimens had crack and light-cure glass ionomer specimens had pores, it was observed that this crack and pores was observed in the control group (self-cure and light-cure glass ionomer of zirconium oxide paericles), but with addition the amount of zirconium oxide particles, the amount of cracking, the number and size of the pores increased, which could be due to the large size of the zirconium oxide particles and the incompatibility with the matrix and poor interface between the matrix and zirconium oxide particles.
Conclusion
Under the limitations of this study, the results showed that the addition of zirconium oxide particles to light-cure glass io
Conflict of Interest
The authors disclose that they have no conflicts of interest.
REFERENCES
- Lohbauer U. Dental glass ionomer cements as permanent filling materials?–Properties, limitations and future trends. Materials. 2009;3:76-96.
- Heymann HO, Swift Jr EJ, Ritter AV. Sturdevant's art and science of operative dentistry. 6th ed, Elsevier Health Sciences; 2014;211-216.
- Aranha AM, Giro EM, Souza PP, Hebling J, De Souza Costa CA. Effect of curing regime on the cytotoxicity of resin-modified glass-ionomer lining cements applied to an odontoblast-cell line. Dental Materials. 2006;22:864-869.
- Moshaverinia A, Ansari S, Moshaverinia M, Roohpour N, Darr JA, Rehman I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomaterialia. 2008;4:432-440.
- Crisp S, Wilson AD. Reactions in glass ionomer cements I. Decomposition of the powder. Journal of dental research. 1974;53:1408-1413.
- Choi JY, Lee HH, Kim HW. Bioactive sol–gel glass added ionomer cement for the regeneration of tooth structure. Journal of Materials Science: Materials in Medicine. 2008;19:3287-3294.
- Summitt JB. Fundamentals of operative dentistry: A contemporary approach. 4th ed, Quintessence Pub.; 2006;319-326.
- Mccabe JF. Flexural properties of resin-modified “hybrid” glass-ionomers in comparison with conventional acid-base glass-ionomers. Dental Materials Journal. 1995;14:109-119.
- Summitt JB. Fundamentals of operative dentistry: A contemporary approach. 4th ed, Quintessence Publishing Company; 2006;501-507.
- Basiri M. Studing the flexural strength of six glass ionomer cements. J Dent. 2007;20:118-129.
- Pearson G, Atkinson A. Long-term flexural strength, of glass ionomer cements. Biomaterials. 1991;12:658-660.
- Smith DC. Development of glass-ionomer cement systems. Biomaterials. 1998;19:467-478.
- Anusavice KJ. Phillips materiais dentários: 12th ed, Elsevier Brasil; 2013;129-138.
- Coutinho E, Cardoso M, De Munck J, Neves A, Van Landuyt K, Poitevin A, et al. Bonding effectiveness and interfacial characterization of a nano-filled resin-modified glass-ionomer. dental materials. 2009;25:1347-1357.
- Strassler HE. Glass ionomers for direct-placement restorations. Dental Economics. 2011;5-8.
- Xie D, Brantley W, Culbertson B, Wang G. Mechanical properties and microstructures of glass-ionomer cements. Dental Materials. 2000;16:129-138.
- Souza JC, Silva JB, Aladim A, Carvalho O, Nascimento RM, Silva FS, et al. Effect of Zirconia and Alumina fillers on the microstructure and mechanical strength of dental glass ionomer cements. The Open Dentistry Journal. 2016;10:58-63.
- Haghgoo R, Rezvani M. The effect of adding different amounts of silver nanoparticles on the mechanical properties of modified glass ionomer resin. Journal of Dental Medicine. 2013;26:211-217.
- Mohammadi basir M, Ataei M, Rezvani M The effect of different amounts of hydroxyapatite nanoparticles on the mechanical properties of resin modified glass ionomer. J Dent Sch. 2011;30:216-223.
- Atai M, Pahlavan A, Moin N. Nano-porous thermally sintered nano silica as novel fillers for dental composites. Dent Mater. 2012;28:133-145.
- Forss H, Seppä L, Lappalaimen R. In vitro abrasion resistance and hardness of glass-ionomer cements. Dent Mater.
- Moshaverinia A, Roohpour N, Chee WW, Schricker SR. A review of powder modifications in conventional glass-ionomer dental ce¬ments. J Mater Chem. 2011;21:1319-1328.
- Wang L, D´Alpino PH, Lopes LG, Pereira JC. Mechanical properties of dental restorative material: relative contribution of laboratory test. J Appl Oral Sci. 2003;11:162-167.
- Peutzfeldt A. Restorative materials for the direct technique. In: Roulet JF, DeGrange M. Adhesion: the silent revolution in dentistry. Chicago: Quintessence Publishing; 2000;61-80.
- Dowling AH, Fleming GJ, McGinly EL, Addison O. Improving the standard of the standard for glass inomers: an alternative the compressive fracture strength test for considearations. J Dent. 2012;40:189-201.
- Kleverlaan CJ, van Duinen RN, Feilzer AJ. Mechanical properties of glass ionomer cements affected by curing methods. Dent Mater. 2004;20:45-50.
- Strassler HE. Glass ionomers for direct-placement restorations. Glass Ionomer Cements 2011.
- Ronald Sakaguchi John Powers. Craig's Restorative Dental Materials. 13th Edition, Elsevier;2012;176
- Arci´s RW, López-Macipe A, Toledano M, Osorio E, Rodri´guez-Clemente R, Murtra J, et al. Mechanical properties of visible light-cured resins reinforced with hydroxyapatite for dental restoration. Dental Materials. 2002;18:49-57.
- Elsaka SE, Hamouda IM, Swain MV. Titanium dioxide nanoparticles addition to a conventional glass-ionomer restorative: Influence on physical and antibacterial properties. Journal of dentistry. 2011;39:589-598.
- Gu Y, Yap A, Cheang P, Khor K. Effects of incorporation of HA/ZrO2 into glass ionomer cement (GIC). Biomaterials. 2005;26:593-599.
- Fleming GJ, Farooq AA, Barralet JE. Influence of powder/liquid mixing ratio on the performance of restorative glass-ionomer dental cement. Biomaterials 2003; 24: 4173-4179.





 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.