Efficacy and Safety of using Rivaroxaban with Coronary Artery Disease Patients: Systematic Review and Meta Analysis
1 Department of Medicine, Arabian Gulf University, Manama, Bahrain
2 Department of Debrecen University, Riyadh, Saudi Arabia
3 Department of Medicine, Albaha University, Albaha, Saudi Arabia
4 Department of General Practice, Ibn Sina National College, Jeddah, Saudi Arabia
5 Department of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
6 Clinical Pharmacist at Taif University,Taif, Saudi Arabia
7 Obs/Gyn Resident at MCH, Tabuk, Saudi Arabia
8 General Practitioner at MOH, Makkah, Saudi Arabia
9 General Practitioner, Qatif, Saudi Arabia
10 Family Medicine Specialist at Alhada armed Forces Hospital, Taif, Saudi Arabia
Published: 30-Dec-2021
Citation: Alharbi SA, et al. Efficacy and Safety of using Rivaroxaban with Coronary Artery Disease Patients: Systematic Review and Meta-Analysis. Ann Med Health Sci Res. 2021;11:5-9.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Rivaroxaban, an oral thing Xa inhibitor, is effective in treating venous thromboembolism, and has been shown to save you thromboembolic activities in atrial fibrillation. Low dose rivaroxaban prevents venous thromboembolism after orthopedic surgical procedures and acute coronary syndrome. Aim: This work aims to determine the efficacy and safety of using rivaroxaban with Coronary Artery Disease (CAD) patients. Materials & Methods: A systematic search was performed over different medical databases to identify Internal Medicine studies, which studied the outcome of the rivaroxaban group versus the Placebo group of CAD patients. We conducted a meta-analysis process on primary efficacy endpoint outcomes (death, Myocardial Infarction “MI”, stroke, or severe recurrent ischemia requiring revascularisation), and on secondary safety endpoint outcomes (major, minor bleeding or bleeding requiring medical attention “TIMI”). Results: Six studies were identified involving 61928 patients, with 34172 patients in the rivaroxaban group, and 27756 patients in the placebo group. Our meta-analysis process showed a highly significant decrease in efficacy endpoint outcomes (death, Myocardial Infarction “MI”, stroke, or severe recurrent ischemia requiring revascularisation), in the rivaroxaban group compared to the placebo group (p<0.001), but also, we found a highly significant increase in safety endpoint outcomes (major, minor bleeding or bleeding requiring medical attention”TIMI”), in the rivaroxaban group compared to the placebo group (p<0.001). Conclusion: To conclude, rivaroxaban may provide an additional therapeutic choice for secondary prevention in patients with CAD. However, to accurately assess the risk of cardiovascular ischemic and bleeding events, we need further studies to gain benefit from rivaroxaban use.
Keywords
Rivaroxaban; artery disease; Endpoints; Venous thromboembolism
Introduction
Stroke is a devastating occurrence in patients with coronary Heart Failure with Reduced Ejection (HFrEF). The sequelae of stroke include a marked decline in health-associated great of lifestyles, better healthcare usage, and increased value of care. Even though Atrial Fibrillation (AF) has been the conventional target populace for stroke threat reduction, patients with HF and sinus rhythm face multiplied chances of stroke compared with the general population. Important gaps exist in our contemporary understanding of stroke threat in this unique population, since prior studies used ancient information, relied on administrative claims facts, and did now not consist of patients on current guideline-mandated scientific therapies. [1]
The cardiovascular results for people the usage of anticoagulant techniques (COMPASS) trial established that in patients with chronic Coronary Artery Disorder (CAD) or Peripheral Artery Disease (PAD) receiving optimal secondary prevention therapies, rivaroxaban 2.5 mg twice daily and aspirin 100 mg once daily in comparison with aspirin 100 mg once day by day decreased the composite of Cardiovascular (CV) demise, stroke or Myocardial Infarction (MI) by 24%, and mortality by way of 18%. [2] Rivaroxaban, an oral thing Xa inhibitor, is effective in treating venous thromboembolism and has been shown to save you thromboembolic activities in atrial fibrillation.
Low dose rivaroxaban prevents venous thromboembolism after orthopedic surgical procedure, and the acute coronary syndrome-thrombolysis in myocardial infarction 51 (ATLAS-2) trial 16 confirmed that low-dose rivaroxaban (2.5 mg two times an afternoon) used in addition to dual antiplatelet remedy decreased predominant damaging cardiovascular activities in patients with acute coronary syndromes, although rivaroxaban by myself (five mg twice an afternoon) extended major, intracranial, and fatal bleeds. Within the COMPASS trial, we sought to become aware of whether a low dose of rivaroxaban given twice an afternoon while used with aspirin or without aspirin, changed into more effective than aspirin by me in lowering important unfavorable cardiovascular activities and important adverse limb activities in patients with peripheral artery disease. [3] This work aims to determine the efficacy and safety of using rivaroxaban with Coronary Artery Disease (CAD) patients.
Materials and Methods
Our review came following the (PRISMA) statement guidelines. [4]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing CAD patients. The excluded studies were either animal or non-English studies or articles describing patients who essentially need rivaroxaban, e.g. pulmonary embolism.
Study identification
Basic searching was done over the pubmed, cochrane library, and google scholar using the following keywords: rivaroxaban, coronary artery disease, Endpoints.
Data extraction
Comparative studies, clinical trials, and Randomized Controlled Trials (RCTs), which studied the outcome of the rivaroxaban group versus Placebo group of CAD patients, will be reviewed. Outcome measures included primary efficacy endpoint outcome (death, Myocardial Infarction “MI”, stroke, or severe recurrent ischemia requiring revascularisation), and secondary safety endpoint outcome (major, minor bleeding, or bleeding requiring medical attention “TIMI”).
Study selection
We found 70 records, 45 excluded because of the title; 25 articles are searched for eligibility by full-text review; 9 articles cannot be accessed; 7 studies were reviews and case reports; the desired drug not used in 3 studies. The studies which met all inclusion criteria were 6 studies.
Statistical analysis
Pooled Odds Ratios (OR), Proportions (%), with 95% Confidence Intervals (CI) assessed, using a statistical package (MedCalc, Belgium). The meta-analysis process was established via I2-statistics (either the fixed-effects model or the random-effects model), according to the Q test for heterogeneity.
Results
The included studies were published between 2009 and 2021. Regarding patients’ characteristics, the total number of patients in all the included studies was 61928 patients, with 34172 patients in the rivaroxaban group, and 27756 patients in the placebo group, while their average follow-up time was (16.2 months). The mean age of all patients was (66 years) [Table 1]. Our meta-analysis included 6 studies comparing 2 different groups of patients; with a total number of patients (N=61928) [Table 2]. [5-9]
| N | Author | Number of patients | Age | Follow-up time | ||
|---|---|---|---|---|---|---|
| Total | Rivaroxaban group | Placebo group | (average years) | (average months) | ||
| 1 | Mega et al. [5] | 3491 | 2331 | 1160 | 57.5 | 6 |
| 2 | Mega et al. [6] | 15526 | 10350 | 5176 | 61.5 | 13.1 |
| 3 | Ohman et al. [7] | 3037 | 1519 | 1518 | 62.5 | 11 |
| 4 | Connolly et al. [8] | 16574 | 8313 | 8261 | 69 | 23 |
| 5 | Zannad et al. [9] | 5022 | 2507 | 2515 | 77 | 21 |
| 6 | Liang et al. [2] | 18278 | 9152 | 9126 | 68.5 | 23.1 |
Table 1: Patients and study characteristics.
| N | Author | Primary outcome | Secondary outcome | ||
|---|---|---|---|---|---|
| Reaching efficacy endpoints | Reaching safety endpoints | ||||
| Rivaroxaban group | Placebo group | Rivaroxaban group | Placebo group | ||
| 1 | Mega | 126 | 79 | 262 | 37 |
| 2 | Mega et al. | 626 | 376 | 147 | 19 |
| 3 | Ohman et al. | 76 | 72 | 80 | 74 |
| 4 | Connolly et al. | 347 | 460 | 263 | 158 |
| 5 | Zannad et al. | 626 | 658 | 18 | 23 |
| 6 | Liang et al. | 379 | 496 | 1076 | 646 |
Table 2: Summary of outcome measures in all studies.
Each outcome was measured by
Odds Ratio (OR):
•For reaching primary efficacy endpoints.
•For reaching secondary safety endpoints.
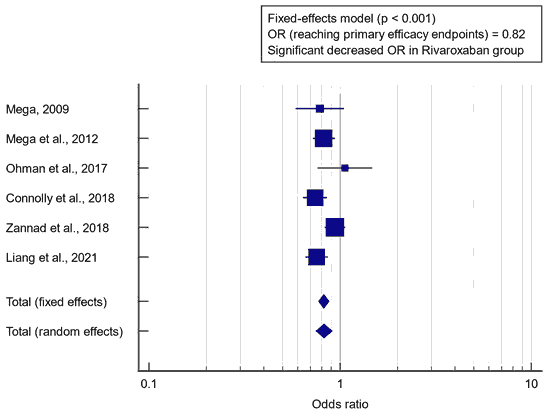
Concerning the primary outcome measure, we found 6 studies that reported reaching the primary efficacy endpoint. I2 (inconsistency) was 51.8%, Q test for heterogeneity (p>0.05), so fixed-effects model was carried out; with overall OR=0.82 (95% CI=0.771 to 0.876). Fixed-effects model of the meta-analysis process revealed a highly significant decrease in efficacy endpoint outcomes (death, Myocardial Infarction “MI”, stroke, or severe recurrent ischemia requiring revascularisation), in the rivaroxaban group compared to the placebo group (p<0.001) [Figure 1].
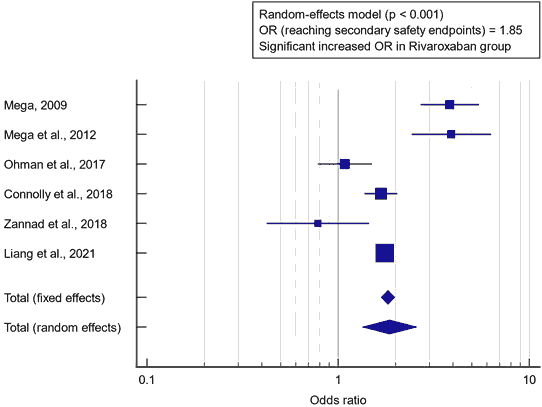
Concerning the secondary outcome measure, we found 6 studies reported reaching secondary safety endpoint. I2 (inconsistency) was 89%, Q test for heterogeneity (p<0.0001), so random-effects model was carried out; with overall OR=1.85 (95% CI=1.337 to 2.586). The random-effects model of the meta-analysis process revealed a highly significant increase in safety endpoint outcomes (major, minor bleeding, or bleeding requiring medical attention “TIMI”), in the rivaroxaban group compared to the placebo group (p<0.001) [Figure 2].
Discussion
The included studies were published between 2009 and 2021. Regarding patients’ characteristics, the total number of patients in all the included studies was 61,928 patients, with 34,172 patients in the rivaroxaban group, and 27,756 patients in the placebo group, while their average follow-up time was (16.2 months). The mean age of all patients was (66 years) which came in agreement with Connolly et al., 2018. [8] Connolly et al., 2018 reported that of the total patients enrolled, 24,824 (91%) had coronary artery disease and were randomised to treatment. The mean follow-up was 1.95 years. The mean age was 68.3 years and 19,792 (80%) were males. [8] Our meta-analysis included 6 studies comparing 2 different groups of patients; with a total number of patients (N=61,928). Concerning the primary outcome measure, we found 6 studies reported reaching the primary efficacy endpoint. Fixed-effects model of the meta-analysis process revealed a highly significant decrease in efficacy endpoint outcomes (death, Myocardial Infarction “MI”, stroke, or severe recurrent ischemia requiring revascularization), in the rivaroxaban group compared to the placebo group (p<0.001) which came in agreement with Xie et al.; Yuan et al.; Zannad et al.; Cowie et al.; Yasuda et al.; Anand et al. [10-14,3]
Xie et al. reported that pooled analysis indicated that the addition of rivaroxaban appreciably decreased the prevalence of the primary efficacy endpoint (RR, 0.86; p=0.01). But, the addition of rivaroxaban became related to a considerably better hazard of the number one safety endpoint (RR, 1.83; p=0.02). [10] Yuan, 2018 reported that 4 trials with a total range of 40,148 patients were covered (23,231 contributors have been treated with rivaroxaban while 16,919 contributors had been treated with placebo) on this analysis. Patients’ enrollment duration was numerous from years 2006 to 2016. The current outcomes showed the addition of rivaroxaban to significantly decrease composite endpoints (OR: 0.81, p<0.001). Similarly, all-cause loss of life, cardiac death, myocardial infarction, and stent thrombosis had been additionally appreciably decreased (OR: 0.82, p<0.001), (OR: 0.8; p=0.002), (OR: 0.87, p=0.03) and (OR: 0.73, p=0.03) respectively. [11]
Zannad et al. reported that, over an average follow-up duration of 21.1 months, the primary endpoint befell in 626 (25%) of 2,507 patients assigned to rivaroxaban and in 658 (26.2%) of 2,515 sufferers assigned to placebo (risk ratio, 0.94, p>0.05). No great difference in all-motive mortality became mentioned among the rivaroxaban institution and the placebo institution (21.8% and 22.1%, respectively; RR, 0.98). The primary safety outcome occurred in 18 patients rivaroxaban patients and in 23 placebo patients (hazard ratio, 0.8; P>0.05). [12] Cowie et al. reported that the rates of non-fatal MI and non-fatal IS were lower for rivaroxaban 2.5 mg bid in combination with aspirin (0.233% and 0.086% vs. 0.253% and 0.159%, respectively), while the price of non-deadly ICH was higher in comparison with aspirin by myself (0.05% vs. 0.019%). Further to a 6% reduction in CV mortality rivaroxaban 2.5 mg bid in combination with aspirin become associated with a longer occasion-free length (14 years vs. 12.7 years). In terms of the wide variety of health events, greater predominant non-deadly more cranial ISTH bleeding events have been simulated with rivaroxaban 2.5 mg bid in mixture with aspirin, while a discount in ALI activities, amputations, and VTE events was observed. [13]
Yasuda et al. reported that the AFIRE was carried out in patients ≥ 20 years with Non-Valvular AF (NVAF) and CAD. Sufferers who have undergone PCI or coronary artery bypass graft at least 1 year previous to enrollment, or the ones without significant coronary lesions requiring PCI (≥ 50% stenosis), could be protected. Approximately 2,200 contributors might be randomized to acquire rivaroxaban monotherapy and rivaroxaban plus an antiplatelet drug (aspirin, clopidogrel, or prasugrel). The primary efficacy endpoints are the composite of cardiovascular events (stroke, non-critical anxious system embolism, myocardial infarction, and risky angina pectoris requiring revascularizations) and all-cause mortality. The primary protection endpoint is essential bleeding as defined via the worldwide society on thrombosis and haemostasis criteria. [14] Anand et al. reported that the combination of rivaroxaban plus aspirin compared with aspirin alone reduced the composite endpoint of cardiovascular death, myocardial infarction, or stroke (5% vs. 7%; HR 0.72, p<0.05), and major adverse limb events including major amputation (1% vs. 2%; HR 0.54 p<0.001). [3]
Concerning the secondary outcome measure, we found 6 studies reported reaching the secondary safety endpoint. The random-effects model of the meta-analysis process revealed a highly significant increase in safety endpoint outcomes (major, minor bleeding, or bleeding requiring medical attention” TIMI”), in the rivaroxaban group compared to the placebo group (p<0.001) which came in agreement with Yuan et al.; and Xie et al. [10,11] Yuan et al. reported that TIMI which was defined as minor and major bleeding to be significantly higher with rivaroxaban (OR: 2.27; p<0.001) and (OR: 3.44 p<0.05) respectively. [11]
Xie et al. reported that primarily based on the outcomes of published trials, the risk of bleeding with including Xa component inhibitors to APT was associated with the dosage. They have a look at ATLAS ACS-TIMI 4617 is a dose-escalation trial and 86.8% of patients obtained rivaroxaban higher or identical to ten mg per day (45.3% 10 mg every day, 22.1% 15 mg each day, and 19.4% 20 mg day by day). Results show the threat of the primary safety endpoint with rivaroxaban increased in a dose-dependent manner. [10]
Conclusion
To conclude, rivaroxaban may provide an additional therapeutic choice for secondary prevention in patients with CAD. However, to accurately assess the risk of cardiovascular ischemic and bleeding events, we need further studies to gain benefit from rivaroxaban use.
Acknowledgment
All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
References
- Mehra MR, Vaduganathan M, Fu M, Ferreira JP, Anker SD, Cleland JG, et al. A comprehensive analysis of the effects of rivaroxaban on stroke or transient ischaemic attack in patients with heart failure, coronary artery disease, and sinus rhythm: The COMMANDER HF trial. Eur Heart J. 2019;40:3593-3602.
- Liang Y, Zhu J, Liu L, Anand SS, Connolly SJ, Bosch J, et al. Efficacy and safety of rivaroxaban plus aspirin in women and men with chronic coronary or peripheral artery disease. Cardiovasc Res. 2021;117:942-949.
- Anand SS, Bosch J, Eikelboom JW, Connolly SJ, Diaz R, Widimsky P, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: An international, randomised, double-blind, placebo-controlled trial. 2018;391:219-229.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Bmj 2009;339.
- Mega JL. ATLAS ACS-TIMI46 study group: Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI46): A randomized, double-blind, phase II trial. 2009;374:29-38.
- Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9-19.
- Ohman EM, Roe MT, Steg PG, James SK, Povsic TJ, White J, et al. Clinically significant bleeding with low-dose rivaroxaban versus aspirin, in addition to P2Y12 inhibition, in Acute Coronary Syndromes (GEMINI-ACS-1): A double-blind, multicentre, randomised trial. 2017;389:1799-1808.
- Connolly SJ, Eikelboom JW, Bosch J, Dagenais G, Dyal L, Lanas F, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: An international, randomised, double-blind, placebo-controlled trial. 2018;391:205-218.
- Zannad F, Anker SD, Byra WM, Cleland JG, Fu M, Gheorghiade M, et al. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med. 2018;379:1332-1342.
- Xie C, Hang Y, Zhu J, Li C, Jiang B, Zhang Y, et al. Benefit and risk of adding rivaroxaban in patients with coronary artery disease: A systematic review and meta-analysis. Clin Cardiol. 2021;44:20-26.
- Yuan J. Efficacy and safety of adding rivaroxaban to the anti-platelet regimen in patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. BMC Pharmacol Toxicol. 2018;19:1-9.
- Zannad F, Anker SD, Byra WM, Cleland JG, Fu M, Gheorghiade M, et al. Rivaroxaban in patients with heart failure, sinus rhythm, and coronary disease. N Engl J Med. 2018;379:1332-1342.
- Cowie MR, Lamy A, Levy P, Mealing S, Millier A, Mernagh P, et al. Health economic evaluation of rivaroxaban in the treatment of patients with chronic coronary artery disease or peripheral artery disease. Cardiovasc Res. 2020;116:1918-1924.
- Yasuda S, Kaikita K, Ogawa H, Akao M, Ako J, Matoba T, et al. Atrial fibrillation and ischemic events with rivaroxaban in patients with stable coronary artery disease (AFIRE): Protocol for a multicenter, prospective, randomized, open-label, parallel group study. Int J Cardiol. 2018;265:108-112.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.