Estimation of Salivary Advanced Oxidation Protein Products Levels in Dental Fluorosis with Chronic Periodontitis Patients
2 Consultant Periodontist, Mumbai, Maharashtra, India
Citation: Warad SB, et al. Estimation of Salivary Advanced Oxidation Protein Products Levels in Dental Fluorosis with Chronic Periodontitis Patients. Ann Med Health Sci Res. 2020;10:1134-1137.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: In normal physiology, there is a dynamic equilibrium between reactive oxygen species (ROS), activity and antioxidant defense capacity and when that equilibrium shifts in favor of ROS, oxidative stress results which leads to periodontal diseases. Advanced oxidation protein products (AOPP) are proteins, predominantly albumin and its aggregates which are damaged by oxidative stress. Fluoride ion at molecular level is related to the production of free radicals and an alteration of the antioxidant defense mechanism. So a study was conducted to estimate the salivary levels of AOPP among healthy, chronic periodontitis (CP) subjects and chronic periodontitis subjects with dental fluorosis (CP-F). Methods: Total 90 subjects were selected and divided into three groups based on clinical parameters group I: 30 healthy subjects, group II: 30 C.P subjects, group III: 30 CP-F subjects, Saliva samples were collected and AOPP levels were determined by U V- spectrophotometry at 340 nm. Results: Salivary levels of AOPP were highest among C.P with dental fluorosis compared to C.P and healthy individuals which were statistically significant. Conclusions: Dental fluorosis can act as an adjunct along with AOPP in contemplating it as a diagnostic indicator of oxidative stress in periodontal disease and aid early planning of treatment modalities.
Keywords
Advanced Oxidative Protein Product (AOPP); Chronic Periodontitis (CP); Dental Fluorosis; Saliva
Introduction
Fluoride plays a dual role in human nutrition, preventing dental caries as well as at higher level of ingestion causing dental and skeletal fluorosis. [1] Dental fluorosis is a condition which is said to be present when there is morphologic alteration in the features of a tooth, in the form of discoloration or anatomic malformation which is attributed to the developmental changes induced by excess fluoride levels in plasma at the time of tooth formation. In India itself, an estimated 60 million people are at risk and 6 million people are disabled. [2] Dental fluorosis is known for the changes it induces in the hard tissues of the body but not much attention has been given to its relation with the surrounding periodontal structures. Similarly, even though a limited study does consider the prevalence of periodontitis in fluorosis subjects. [3,4]
Studies suggest that there is a strong association of occurrence of periodontal disease in high-fluoride areas. [5] It also contributes to genetic alterations inducing up-regulation and downregulation of genes. [6] Its influence on MMP-20 is known for it to contribute to dental fluorosis in rats. [7] Considering all this evidence, it can be said that fluorosis has the potential not only to influence the periodontium cytologically but molecularly as well, thereby modifying enzymatic actions as wells as protein formation. Surface roughness, after analyzing with atomic microscopy revealed surface roughness is exceedingly high in fluorosis subjects with roughness increasing with degree of fluorosis. [8] This surface roughness is conducive for the bacteria to survive as well as make it difficult for scaling and root planning in fluorosed teeth. This could also jeopardize the effectiveness of the regular oral hygiene procedures. The increase in ROS induced by NaF suggests that fluoride causes a higher degree of oxidative stress and extensive cellular damage in tissues. [9]
Over the past few years, strong evidence has emerged to implicate oxidative stress in pathogenesis of periodontitis. Free radicals and reactive oxygen species (ROS) are essential to many normal biological processes. At low concentrations, these free radicals stimulate the growth of fibroblast and epithelial cells in culture, but at higher concentrations it may result in tissue injury. [10] Chronic periodontitis (CP) is chronic disease considered as a complex immuno-inflammatory disease characterized by an exaggerated host response leading to enhanced Reactive oxygen species (ROS) production by peripheral neutrophils. Since molecular products from oxidative stress are generally more stable than oxidants themselves, ROS measurements often involve determining levels of their oxidation target products. [11]
Protein oxidation can result in the formation of protein carbonyls, formation of cross linked molecules by sulfhydryl group oxidation and formation of advanced oxidation protein products. [12] In the year 1996, Witko-Sarsat et al. identified novel family of oxidized protein compounds which were termed as advanced oxidation protein products (AOPP). These are dityrosine containing cross linked protein products which makes them different from other protein aggregates that form as a result of disulphide links after a subtle oxidative stress. [13]
These products originate when hypochlorous acid (HOCL) along with other chlorinated oxidants like (OCL-) react with protein compounds. HOCL is formed by the enzymatic action of myeloperoxidase which catalyses the reaction of chloride ion with hydrogen peroxide. [14] AOPP are recognized as markers of oxidative damage to proteins, related to intensity of oxidative stress and inflammation.13 Serum AOPP levels increase with progression of chronic diseases and it has been used as inflammatory marker for liver cirrhosis and chronic renal failure. [14-16]
The well-established relation between AOPP response and inflammation suggests that it is the best marker or monitoring fluoride mediated oxidative stress damage on periodontal structure. To date there have been few studies of the effect of Fluoride on periodontal health. Therefore, the main objective of this research was to investigate the effect of fluoride in the pathogenesis of periodontal disease by quantification of AOPP levels.
Research Methodology
The present cross-sectional study was conducted on 90 individuals of age between 18-60 years, who reported to the outpatient department of Periodontics, P.M.N.M. Dental College & Hospital Bagalkot. The research protocol was reviewed and approved by the Institutional Ethical Committee. A written informed consent was obtained from all the selected patients prior to commencement of the study.
The study group consists of 30 individuals with chronic periodontitis (CP), 30 individuals with chronic periodontitis with fluorosis (CP-F) and 30 healthy individuals (controls).
After clinical examination the subjects were divided in 3 groups. Group I consisted of healthy subjects showing absence of clinical and radiographic manifestations of periodontal disease, at least 20 teeth present with a GI score < 1 and absence of loss of attachment, Group II comprised the subjects diagnosed as chronic periodontitis having minimum of six teeth with periodontal pockets of PD>5 mm & Clinical attachment loss CAL >3 mm & GI>Group III consisted of subjects fulfilling the criteria of group II in addition to having dental fluorosis (dean’s criteria mild to severe)
Exclusion criteria included subjects with history of any systemic diseases or conditions and with any apparent oral infections (i.e., Herpes or Candida). Any injuries or bleeding in the oral cavity unrelated to gingivitis or periodontitis, intake of antibiotics or anti-inflammatory drugs within 6 months prior to the study were excluded from the study. Pregnant or lactating women, subjects with history of smoking or any form of tobacco and alcohol consumption and individuals who had received any periodontal treatment in the past six months prior to the study were also excluded from the study.
Method of saliva collection
Subjects were instructed not to eat or drink one hour before the time of saliva collection. Patients were explained about the study and required appointment was given for saliva sample collection. Unstimulated whole saliva was collected between 10am and 12 Noon to avoid diurnal variation. [17] Patients were seated on dental chair and were instructed to rinse with water to void of the previously collected saliva. The patients were asked to allow saliva to accumulate in the floor of the mouth for a minute and to spit without stimulation into the screw top tube. Prior to analysis, saliva samples were centrifuged at 4000 rpm for 10 min. The supernatant fraction was then aliquotted into storage vials and kept at -800C until required for biochemical analysis. [18]
Biochemical analysis
Salivary AOPP levels determined using a spectrophotometric method given by Witko-Sarsat et al. Briefly 200 μL of saliva was incubated with 20 μL of glacial acetic acid. The absorbance was read immediately at 340 nm. AOPP concentration was expressed in μmol/ml on the basis of the calibration curve of chloramine-T with potassium iodide. [16]
Statistical analysis
Statistical analyses were performed, using Kruskal Wallis test and Mann Whitney U test. It showed a significant p-value (P<0.05). The statistical significance of correlations among variables was determined using the Spearman rank correlation coefficient test.
Results and Discussion
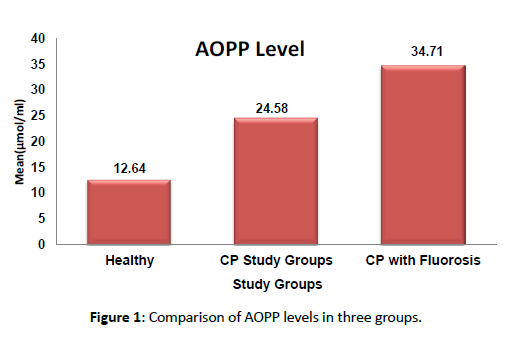
All the samples in each group, tested positive for AOPP and the study showed an increased concentration of salivary AOPP in chronic periodontitis with fluorosis patients and chronic periodontitis patients compared to healthy individuals which were statistically significant with p<0.05 as shown in Figure 1. When AOPP levels are correlated with clinical parameters a significant positive correlation was found between levels of AOPP with GI, PD, CAL, and Modified deans fluorosis index [Table 1].
Table 1: Comparison of clinical parameters with AOPP levels in three groups.
| AOPP LEVEL µmol/ml | ||||
|---|---|---|---|---|
| Healthy | CP | CP with Fluorosis | ||
| Gingival Index | Correlation Coefficient | 0.92 | 0.98 | 0.96 |
| p-value | <0.001* | <0.001* | <0.001* | |
| Probing Depth | Correlation Coefficient | -0.06 | 0.77 | 0.78 |
| p-value | 0.75(NS) | <0.001* | <0.001* | |
| Clinical Attachment Loss | Correlation Coefficient | - | 0.80 | 0.83 |
| p-value | - | <0.001* | <0.001* | |
| Modified Deans Fluorosis Index | Correlation Coefficient | - | - | 0.81 |
| p-value | - | - | <0.001* | |
Oxidative stress is a misbalance between the production of free radicals and antioxidant status leading to oxidative damage of macromolecules including lipids and proteins. This imbalance between the ROS-AO has been implicated as one of the progressive or pathogenic factors for periodontal disease. [19]
Previous studies have shown that AOPP plays a dynamic role in complex patho-physiology of oxidative stress and inflammation. But the mechanisms by which AOPP accelerate inflammation remain to be investigated. [20] AOPP is generated by different oxidation patterns, which might lead to production of reactive oxygen species such as hydrogen peroxide. These ROS secondarily leads to increased inflammatory reaction by signaling activation of NF-Kβ which stimulates pro-inflammatory cytokines release through depletion of intracellular thiol compounds. This could possibly explain higher levels of AOPP found in chronic periodontitis groups as compared to healthy individuals. [21,22] Fluoride exposure increase the generation of anion superoxide (O2 -); increased O2 - concentration and its downstream consequences such as hydrogen peroxide, peroxynitrite, hydroxyl radicals seem particularly important in mediating fluoride’s effects. [23,24] Fluoride is thought to inhibit the activity of antioxidant enzymes such as superoxide dismutase (SOD), glutathione peroxidase, and catalase often resulting in the excessive production of ROS at the mitochondrial level, leading to the damage of cellular components. [23-25]
In present study increase in AOPP level was seen in chronic periodontitis with fluorosis group compared to other groups. The findings in our study keep in tract with the observations found by Piwowar A, who described that AOPP formation is induced by intensified glycol-oxidation process, oxidant antioxidant imbalance and coexisting inflammation. [26] So from our study it is reasonably to predict that fluoride causes an increase in oxidative stress and has a role in periodontal disease. As this was the first study determining the levels of AOPP among periodontitis and periodontitis with fluorosis subjects, a direct comparison with other research was not possible. There is a long list of laboratory methods available in the literature that are in use in establishing the presence of oxidative stress, but none of them proved to be unequivocally superior to the others until now. Sometimes they are used in combination with each other, but financial considerations strongly limit this practice in routine diagnostics. AOPP are just one of those techniques. It is suggested that the AOPP shows immense importance as an oxidative stress biomarker. [27]
The well-established relationship between AOPP response and induced damage makes this simple, fast and inexpensive technique applicable in daily routine practice for measuring oxidative stress mediated damage in chronic inflammatory conditions. The results of this research lay emphasis on the need for studies with large sample size to authenticate the impact of fluoride on periodontal disease and AOPP Levels. We only hope that these studies will encourage further research in this direction and help in better understanding of the relationship between the fluorosis and periodontal disease.
Conclusion
It is concluded from our study that there is direct association between high AOPP levels in fluorosis subjects and periodontal disease. Fluoride causes dental and skeletal changes in people residing in endemic fluorosis area so action needs to be taken to counteract these problems. Increased AOPP levels could be used as early diagnostic marker to predict the activity and severity of the disease. The simple chair side test for AOPP can be a valuable tool in periodontal disease diagnosis and early planning of treatment modalities.
Competing Interests
The authors declare that they have no competing interests.
REFERENCES
- Salah H, Arab N. Application of PIGE to determine fluorine concentration in human teeth: Contribution to fluorosis study. J Radioanal Nucl Chem. 2007;8:31-34.
- Reddy DR. Neurology of endemic skeletal fluorosis. Neurol India. 2009;57:7-12.
- Reddy J, Parker JP, Africa CW, Stephen LX. Prevalence and severity of periodontitis in a high fluoride area in South Africa. Community Dent Oral Epidemol. 1985;13:108-112.
- Grembowski D, Fiset L, Spadafora A, Milgrom P. Fluoridation effects on periodontal disease among adults. J Periodont Res. 1993;28:166-172.
- Vandana KL, Reddy MS. Assessment of Periodontal Status in Dental Fluorosis Subjects using Community Periodontal Index of Treatment Needs. Indian J Dent Res. 2007;18:167-171.
- Vazirani SJ, Sing A. Endemic dental fluorosis, radiological features of dental fluorosis. J Indian Dent Assoc. 1968;40:299-303.
- Krook L, Maylin GA, Lillie JH, Wallace RS. Dental fluorosis in cattle. Cornell Vet. 1983;73:340-362.
- Susheela AK, Bhatnagar M, Gnanasundram N, Saraswathy TR. Structural aberrations in fluorosed human teeth: biochemical and scanning electron microscopic studies. Curr Sci. 1999;77:l-6.
- Das J, Ghosh J, Manna P, Sil PC. Taurine provides antioxidant defense against NaF induced cytotoxicity in murine hepatocytes. Pathophysiology. 2008;15:181-190.
- Parveen D, Reet K, Rajan G, Rohit B, Karun C, et al. Reactive oxygen species in periodontitis. J Indian Soc Periodontol. 2013;17:411-416.
- Sharma A Sharma S. ROS and anti-oxidants in periodontics: A review. Int J Clin Dent. 2011;3:44-47.
- Veyseller B. Aksoy F, Ertas B, Keskin M, Ozhuran O, Yillldrium YS. A new oxidative stress marker in patients with nasal polyposis: advanced oxidation protein products (AOPP). B-ENT 2010:6:105-109.
- Witko-Sarsat V, Friedlander M, Khoa TN, Capeillère-Blandin C, Nguyen AT, Canteloup S, et al. Advanced Oxidation Protein Products as Novel Mediators of Inflammation and Monocyte Activation in Chronic Renal Failure. J Inflammation. 1998;161:2524-2532.
- Baskol M, Baskol G, Kocer D, OzbakirO, Yucesoy M. Advanced Oxidation Protein Products A Novel Marker of Oxidative Stress in Ulcerative Colitis. J Clin Gastroenterol. 2008;42:687-691.
- Jagiello JZ, Simon MP, Simon K, Warwas M. Elevated advanced oxidation protein products levels in patients with liver cirrhosis. J Acta Biochemica Polonica. 2009;56:679-685.
- Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Init.1996;49:1304-1313.
- Kaufman E, Lamster IB. Analysis of saliva for periodontal diagnosis: a review. J Clin Periodontol 2000;27:453-465.
- Navazesh M. Method for collecting saliva. Ann. NY. Acad. 1994;694:72-77.
- Waddington R, Moseley R, Embery G. Periodontal Disease Mechanisms: Reactive oxygen species: a potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138-51.
- Liu SX, Hou FF, Guo ZJ, Nagai R, Zhang WR, Liu ZQ, et al. Advanced oxidation protein products accelerate atherosclerosis through and promoting oxidative stress and inflammation. Arterio sclera thromb vasc biol. 2006;26:1156-1162.
- Chaudhary S, Gowda TM, Mehta, DS, Kumar T. Comparative evaluations of plasma ROM levels in chronic periodontitis patients before and after non-surgical and surgical periodontal therapy: A clinical trial. J Indian Soc Periodontol. 2014;18:140-144.
- Servettaz A, Guilpain P, Goulvestre C, Chereau C, Hercend C, Nicco C, et al. Radical oxygen species production induced by advanced oxidation protein products predicts clinical evolution and response to treatment in systemic sclerosis. Ann Rheum Dis. 2007;66:1202-1209.
- García-Montalvo EA, Reyes-Pérez H, Del Razo LM. Fluoride exposure impairs glucose tolerance via decreased insulin expression and oxidative stress. Toxicology. 2009;263:75-83.
- Izquierdo-Vega JA, Sánchez-Gutiérrez M, Del Razo LM. Decreased in vitro fertility in male rats exposed to fluoride-induced oxidative stress damage and mitochondrial transmembrane potential loss. Toxicol Appl Pharmacol. 2008;230:352-357.
- Nobes CD, Hall A. RHO, RAC, and CDC-42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53-62.
- Piwowar A. Advanced oxidation protein products. Part II. The significance of oxidation protein products in the pathomechanism of diabetes and its complications. Pol Merkur Lekarski. 2010;28:227-230.
- Selmeci L. Advanced oxidation protein products (AOPP): Novel remic toxins, or components of the non-enzymatic antioxidant system of the plasma proteome? Free Radic Res. 2011;45:1115-1123.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.