Evaluation of the Analgesic Activity of the Methanolic Stem Bark Extract of Dialium Guineense (Wild)
- *Corresponding Author:
- Dr. M.I. Ezeja
Department of Veterinary Physiology, Pharmacology and biochemistry, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria. Tel: +2348033238975
E-mail: ezejamaxwell@yahoo.com
Date of Received :21/07/2010
Date of revised :24-07-2011
Date of accepted :10/08/2010
Abstract
Background: Dialium guineense is a medicinal plant used by some communities of Enugu-Ezike in Enugu State, Nigeria for treatment of fever, headache and other diverse ailments.
Objectives: The present study evaluated the analgesic activity of the methanolic stem bark extract of the plant.
Method: Acetic acid-induced abdominal constriction or writhing, tail immersion and hot plate analgesic models in albino Wistar mice were used for the study. Three test doses (250, 500, 1000 mg/kg body weight) of the extract were administered orally by gastric gavage. The activity was compared with a standard reference drug, acetylsalicylic acid (aspirin) (400 mg/kg) and negative control. The results were analysed by SPSS version 17 using ANOVA and Post Hoc Duncan.
Result: In the acetic acid-induced writhing reflex model, D. guineense extract and the reference drug significantly (P =0.014 - 0.002) decreased the mean total number of abdominal constriction in the mice in a dose dependent fashion. The percentage inhibition of the abdominal constriction reflex was increased dose dependently from 0% in the negative control group to 71% at the highest dose of the extract (1000mg/kg). In the tail immersion model the extract at the dose of 1000 mg/kg significantly (P = 0. 048) increased the pain reaction time (PRT) while in hot plate model the extract and drug also significantly (P = 0.048 - 0.05) increased the mean PRT at the doses of 500 and 1000 mg/kg. The dose of 250 mg/kg showed no analgesic activity in tail immersion and hot plate models.
Conclusion: Dialium guineense demonstrated significant analgesic activity that may be mediated through peripheral pain mechanism
Key words
Dialium guineense; acetylsalicylic acid; writhing; hot plate: PRT.
Introduction
Pain is a disabling accompaniment of many medical conditions and pain control is one of the most important therapeutic priorities.[1] Pain has been officially defined as an unpleasant sensory and emotional experience associated with actual or potential tissue damage. It is always a warning signal and primarily protective in nature but often causes a lot of discomfort and lead to many adverse effects.[2] Analgesics are drugs used to treat or reduce pain and the classical analgesic drugs notably opiates and non-steroidal antiinflammatory drugs have their origin in natural products but many synthetic compounds that act by the same mechanism have been developed and are associated with serious adverse effects such as ulceration, gastrointestinal bleeding, additive potential, respiratory distress, drowsiness, nausea etc .[3, 4].
Based on these therefore, there is the need for the search for bioactive compounds from natural products especially from medicinal plants for use as alternative analgesics with little or no side effects.
Dialium guineense (Wild) belongs to the family of Fabaceae. It is a small tree or shrub 10-20 m high with dense crown and hanging leaves. The bark is smooth, greyish, and slash-reddish sometimes exuding a red gum. Stems pubescent and brown. The leaves are alternate usually with 5-7 opposite or sub alternate leaflets. The flowers are green about 10cm across and5-6 mm in diameter. The fruits are lenticular or flattened globose, about 2-2.5cm in diameter containing 1and sometimes 2 seeds embedded in a reddish acidulous and sweetly edible pulp.[5]
It grows in dense savannah forests, shadowy canyons and gallery forests. It is found in Senegal to Sudan along the Southern border of the Sahel and it is the most common and widespread Dialium in Nigeria and also found in Ghana along transition zone. In Nigeria, the tree flowers from September to October and fruits from October to January.[6]
Other common names include: black velvet or velvet tarimand (English), Icheku (Ibo, Eastern Nigeria), Awin (Yoruba. Western Nigeria), Tamarinier noir (French).[7]
Different parts of the tree have been used in folkloric medicine for treatment of different diseases such as cancer, headache and pains (bark), fever, prenatal pains and oedema (leaves), diarrhoea (fruits).[5]
The present study was therefore undertaken to investigate the analgesic activity of the stem bark of Dialium guineense with the aim of establishing the pharmacological basis for its folkloric use to treat pains and headaches.
Materials and Methods
Plant collection and Identification
The stem bark of Dialium guineense was collected from the premises of University of Agriculture, Umudike Abia State and identified as Dialium guineense (Wild) by Dr. I. Dike of forestry department of the University and a voucher specimen number VPP/HB/056/2010 was deposited in the department of Veterinary Physiology, Biochemistry and Pharmacology herbarium.
Preparation of Plant Extract
The stem bark of Dialium guineense was collected and chopped into small pieces with a knife, dried under a mild sunlight and pulverised into a coarse powder of about 1mm in diameter. The extraction was done by cold maceration method in 80% methanol for 48hrs with intermittent shaking at 2 hrs interval. Later, the extract was filtered with Whatman No1 filter papers and the filtrate concentrated to dryness in an oven at 400 C. The percentage yield was calculated using the formula below and the extract stored in a refrigerator at 150 C until time of use.

Animals
75 Albino Wistar mice weighing 21-30 g obtained from laboratory animal units of the faculty of Veterinary Medicine, University of Nigeria Nsukka, Enugu State were used for the experiment. The animals were kept in stainless steel cages and housed at an ambient temperature of between 25- 27oc and relative humidity of about 50-55% with free access to feed (Vital feed®, Nigeria) and water. Ethical guidelines governing the use of live animals for conduct of experiments as stipulated by Zimmerman8 was strictly followed and the study protocol was approved by the Michael Okpara University"s ethical committee.
Acute Toxicity Test
The method of Lorke 9was used for this study. Twenty five mice of both sexes were randomly grouped into five groups (A-E) of five mice each. Groups A, B, C, D and E mice were dosed with 100, 500, 1000, 2000 and 3000 mg/kg respectively orally by gastric gavage. The animals were given free access to feed and water. They were observed over a period of 24 hours for signs of toxicity and mortality.
Acetic Acid Writhing Reflex Method
This study was carried out using the method of Koster [10] as modified by Danbisya and Lee[11]. Twenty five albino mice of both sexes were randomly divided into five groups (A-E) of five mice per group. They were fasted for 12 hours and later treated as follows: Group A mice were given tween 20 solution 10 ml/kg (negative control group), group B mice were given 400mg/kg acetylsalicylic acid (Aspirin) (positive control group) while groups C, D and E received 250, 500, 1000 mg/kg of Dialium guineense extract respectively all by gastric gavage. 1 hour after administration of drug and extract, 0.7% glacial acetic acid (10 ml/kg) was given intraperitoneally (i.p) to all the mice to induce pain characterised by abdominal constrictions or writhes. The number of writhes observed in each mouse was counted for 30 minutes and recorded. The percentage protection against abdominal writhing was used to assess the degree of analgesia and was calculated using the formula.[11]
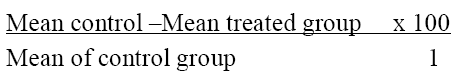
Tail Immersion Test
The method described by Uma-Devi[12] was used for this experiment. Twenty five (25) albino mice were randomly divided into five groups with five mice each, all fasted for 12 hours with clean drinking water provided ad libitum. The animals were pretreated 60 minutes before tail immersion with 10ml/kg tween 20 solution for group A (negative control), 400 mg/kg acetylsalicylate acid (aspirin) for group B (positive control) and 250, 500, 1000 mg/kg of Dialium guineense extract for groups C, D and E respectively. Then about 2-3cm of the tail of each of the mice was dipped into a water bath containing warm water maintained at a temperature of 50 ± 10 C and the time taken for the mice to flick its tail or withdraw it from the warm water known as the pain reaction time (PRT) was recorded for all the mice. The cut off time was put at 15 seconds.
Hot Plate Method
The method described by Shethy and Anika (1982) [13] as modified by Franzotti et al. (2000) [14] was used for this study. Albino mice of both sexes were randomly grouped into five groups of five mice each, fasted for 12 hours with adequate clean water provided ad libitum. Each of the mice was placed on a hot plate maintained at the temperature of 55 ± 10 C and the pain reaction time (PRT) or latency period determined with a stop watch was recorded which represents the time taken for the mice to react to the pain stimulus. The response to pain stimulus considered included; jumping, raising and licking of hind foot. The cut off time was fixed for 20 seconds. This served as control pain reaction time. The mice were then treated as follows: Group A received tween 20 solution (negative control), group B received 400mg/kg
Data Analysis
The result were presented as mean ± SEM and analysed using One way Analysis of Variance (ANOVA). The difference between the means was tested with Post Hoc Duncan and t-test and values of p <0.05 were considered statistically significant.
Aspirin (positive control) while groups C, D and E received 250, 500, 1000 mg/kg of D. guineense extract respectively. Thirty minutes after drug and extract administration, the pain reaction time for each mouse was again determined and recorded using the same method as above.
New set of mice were used for each experiment.
Results
Plant Extraction
The yield of the stem bark extract was 4.25% w/w dry matter and was dark in colour.
Acute Toxicity Test
Acute toxicity test of the extract produced no death or signs of toxicity after 24 hours even at the dose of 3000 mg/kg which shows that the extract was well tolerated.
Acetic Acid Induced Writhing Reflex
The effect of D. guineense extract on the acetic acid- induced abdominal constrictions in mice is presented in Table 1. The result shows that the extract (250, 500, 1000 mg/kg), and the reference drug aspirin (400 mg/kg) significantly (P < 0.0001) reduced abdominal writhing in mice when compared to the negative control group reducing the mean number of writhing from 30.5 ± 7.46 in the negative group to 9.0 ± 4.29 at the dose of 1000 mg/kg. The reduction was in a dose dependent manner. Also the extract caused a dose dependent increase in inhibition of abdominal writhing, increasing it from 0% in negative control group to 71% at the dose 1000 mg/kg. Furthermore, posthoc analysis did not detect any significant difference between the extract at the doses of 250 versus reference drug (aspirin) and control group (P =0.13 and 0.18 respectively).Also no significant differences occurred between 500mg/kg versus reference drug and 1000mg/kg versus reference drug (P =0.22 and 0.31 respectively).

Tail Immersion Method
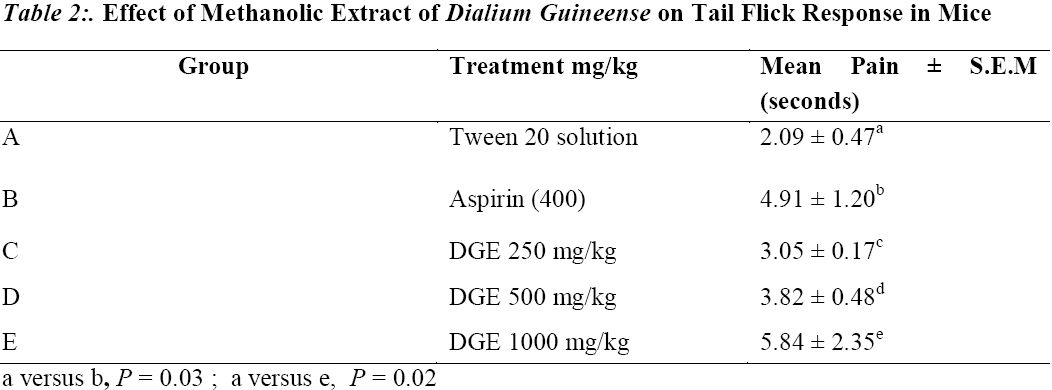
The result of tail immersion test in mice is presented in Table 2. The result shows that the extract at the dose of 1000mg/kg and the reference drug aspirin significantly (P = 0.03 and P = 0.02 respectively) increased the PRT when compared to the negative group (group 1). At the doses of 250 and 500 mg/kg, the extract did not show any significant increase in PRT, although there was a marginal increase in the mean PRT from 2.09 ± 0.47 to 3.82 ± 0.48 (P =0.28 and 0.42 respectively).
Hot Plate Method
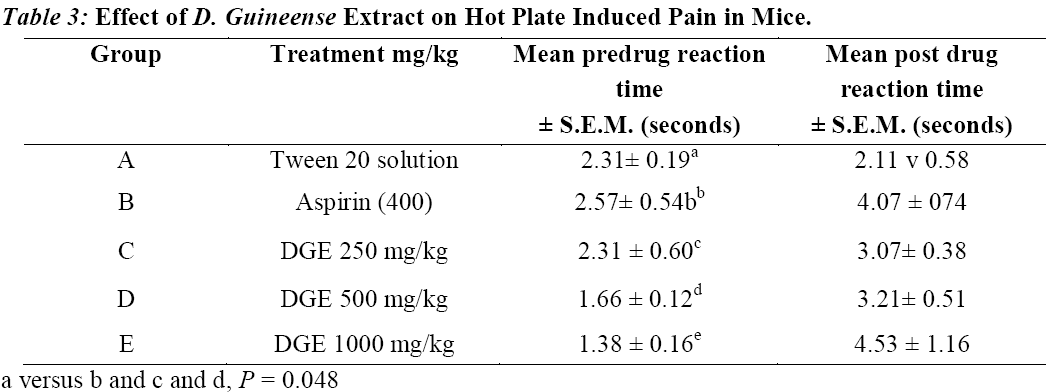
The result of the effect of Dialium guineense on the hot plate method is presented in Table 3. The result shows that there was no significant difference in the PRT during the pre drug testing time. After drug and extract administration, comparing the pre and post drug PRT using T-test showed that the reference drug aspirin (400 mg/kg) and the extract at the doses of 500 and 1000 mg/kg significantly (P = 0.048) increased the PRT with the extract at the dose of 500 mg/kg producing a better effect than the reference drug and the 1000 mg/kg dose. The extract at the dose of 250 mg/kg did not show any significant increase in the mean PRT.
Discussion
Three anti-nociceptive models; acetic acidinduced writhing reflex, tail immersion and hot plate models were used to evaluate the analgesic activity of Dialium guineense since tests of analgesic drugs commonly measure nociception and involves the reaction of animals to painful stimuli.[1] The stimulus may be thermal (tail immersion or hot plate tests), chemical (acetic acid-induced writhing or formalin tests) or mechanical (tail or paw pressure tests).[15] The methanolic stem bark extract of D. guineense produced no death or signs of toxicity even at the dose of 3000 mg/kg which suggests that the extract was well tolerated by the mice and that the doses used were safe.
Acetic acid-induced writhing reflex is a model of visceral pain which is highly useful for screening analgesic drugs [2] and several chemicals such as phenylquinine and acetic acid could induce writhing reflex in laboratory animals.[6] Intraperitoneal injection of 0.7% glacial acetic acid produced abdominal writhing in this experiment. Acetic acid produces writhing reflex in animals by activating the chemo sensitive nociceptors.[17] Also, it has been noted that the level of analgesia in acetic acid-induced models is indicated by the percent reduction in the number of abdominal constrictions.[18] In this experiment, the reference drug and D. guineense extract at 500 and 1000 mg/kg significantly decreased the mean number of abdominal constrictions or writhes which was dose dependent. Also the extract increased the percent inhibition of abdominal constriction from 0% in the negative group to 71% at the dose of 1000 mg/kg. This effect was superior to that of the reference drug aspirin. Acetic acid-induced writhing model produces pain sensation by triggering inflammatory response and such pain stimulus leads to release of arachidonic acid from tissue.[9] The analgesic effect of D. guineense seen in this experiment may be mediated through peripheral pain mechanism and or through suppression of prostaglandin pathway since it has been observed that any agent that decreases the number of writhing will demonstrate analgesia preferably by inhibition of prostaglandin synthesis, a peripheral mechanism of pain inhibition.[20]
The procedure in the tail immersion test is based on the observation that morphine-like drugs selectively prolongs the reaction time of the typical tail withdrawal reflex in mice [21] while in the hot plate model, the paws of mice are very sensitive to temperatures at 50-55 ± 10C.[14] In both models, (tail immersion and hot plate), increase in pain reaction time (PRT) or latency period indicates the level of analgesia of drug or extract.[22]
In the tail immersion, the extract only showed a significant increase in PRT at the dose of 1000 mg/kg when compared to the negative group while in the hot plate model, comparing the PRT pre and post treatment, the extract at the doses of 500 and 1000 mg/kg significantly increased the pain reaction time and the extract at the dose of 500 mg/kg had a better analgesic effect than other groups which was unexpected but may be the maximum dose beyond which the analgesic activity of the extract will no longer be increased in this model.
The tail immersion and hot plate models have been used to study centrally acting analgesics.[23] In these models, sensory nerves sensitise the nociceptors and the involvement of endogenous substances such as prostaglandins are minimised.[24] From the results, though the extract showed analgesic actions in the tail immersion and hot plate models, it was not as pronounced as was seen in the acetic acid-induced model and this may suggest that the analgesic activity of D. guineense may not be fully mediated through central mechanism.
In conclusion, the methanolic stem bark extract of Dialium guineense demonstrated significant analgesic activity and may be acting through inhibition of prostaglandin pathway and or through peripheral pain mechanism. However, more work is required in the isolation and characterisation of the bioactive compound(s) and determination of the exact mechanism of action.
References
- Rang HP, Dale MM, Ritter JM and Moore PK. Pharmacology 5th edn. New Delhi India: Elsevier Science ltd; 2003.
- Raquibul SM, Hossain, MM, Aktar R, Jamila M, Mazumder MEH, Alam MA, et al. Analgesic Activity of the Different Fractions of the Aerial Parts of Commenila Benghalensis Linn. International Journal of Pharmacology 2010; 6(1): 63-67.
- Laurence DR, Benneth PN and Brown MJ. Clinical Pharmacology, 8th edn. Edinburgh: ChurchHill Livingstone; 1997.
- Mate GS, Naikwade NS, Chowki CSA and Patil SB. Evaluation of Anti-nociceptive Activity of Cissus quadrangularis on Albino Mice. Int J Green Pharm 2008; 2: 118-121.
- Arbonnier M. Trees, Shrubs and Lianas of West African dry zones 2nd edn. GMBH: Margaraf publishers; 2004.
- Keay RWJ. Trees of Nigeria, Oxford: Clarendon press; 1989.
- www.worldagroforestrycenter.org/sea/prod ucts (accessed August 20, 2010)
- Zimmermann M. Ethical Guidelines for Investigations of Experimental Pain in conscious Animals. Pain 1983; 16 (2): 109-110.
- Lorke D. A new approach to practical acute toxicity. Archives of Toxicology 1983; 53:275-289.
- Koster R, Anderson M and Debeer EJ. Acetic acid for Analgesic Screening. Federation Proceedings 1959; 18: 412-415.
- Dambisya YM and Lee S. Influence of Temperature, pH and Naloxone on the Anti-nociceptive Activity of Chana striatus (Haraun) Extract in Mice. J Ethnopharmacol 1999; 66: 181-186.
- Uma-Devi P, Ganasounder IA, Rao SB and Srivasan KK. In Vitro Radioprotection by Ocimum flavonoids, Survival of Mice.Radiation Research 1999; 151: 74-78.
- Shetty SN and Anika SM. Laboratory manual of Pharmacology and Toxicology, 1st ed. Enugu: Fourth Dimension Publishers; 1982.
- Franzotti EM, Santos CVF, Rodrigues HMS, Mourao RHV, Andrade MR et al. Anti-inflammatory, analgesic and acute toxicity of Sida cordiforlia L. J Ethnopharmacol 2000; 72:273-277.
- George KA, Eric W, David DO and George AK. Antinociceptive Effects of Newboulia Laveis (P. Beauv) Stem Bark Extract in RatModel. Pharmacog Mag 2009; 17: 49-54.
- Berkenkopf JW and Weichman BM. Production of Protacyclin in Mice following Intraperitoneal Injection of Acetic Acid, Phenylbenzoquine and Zymosan; its role in the Writhing Response. Prostaglandins 1988; 36: 693-709.
- Onasanwo SA and Elegbe RA. Antinociceptive and Anti-inflammatory Properties of the leaf Extract of Hedranthera barteri in Rats and Mice. African J Biomedical Research 2006; 2: 109-118.
- Machioro M, Blank MFA, Moura RHV and Antioniolli AR. Antinociceptive Activity of the Aqueous Extract of Erythrina velutina leaves. Fitoterapia 2005; 76: 637-642.
- Ahmed F, Hossain MH, Rahman AA and Shahid TZ. Anti-nociceptive and sedative effects of the bark of Cerbera odolla. J Oriental Pharmacy Exp. Med 2006; 6:344-348.
- Ferdous M, Rouf R, Shilpi JA and Uddin SJ. Anti-nociceptive activity of the ethanolic extract of Ficus racemosa (Lin). Oriental Pharm Exp Med 2008; 8:93-96.
- Toma WO, Graciosa JS, Hiruma CA, Andrade FDP, Vilegas W and Souza-Brita ARM. Evaluation of the analgesic and anti-edamatogenic activities of Quassia mara barks extract. J Ethnopharmacol 2003; 85: 19-23.
- Ranadran K and Basinath L. A critical Analysis of the Experimental Evaluation of Nociceptive reactions in Animals. Pharm Research 1986; 3: 253-270.
- Woolfe G and MacDonald AD. The evaluation of analgesic action pethidine hydrochloride. J Pharmacol Exp Ther 1994; 80:300.
- Bachlav RS, Gulecha VS and Upasani CD. Analgesic and Anti-inflammatory Activity of Argyreia Speciosa roots. Indian J Pharmacol2009; 41(4): 158-161.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.