Exceptional Response to Radio-Target Therapy in a Case of Metastatic Nodular Melanoma
1 Department of Oncology-Radiotherapy, Institute of Oncology, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania, Email: medicanca@gmail.com
2 Department of Internal Medicine, Clinical Emergency Hospital of Bucharest, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
3 General Surgery Department, Clinical Emergency Hospital, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
, DOI: 10.54608.annalsmedical.2021.16
Citation: Haineala B, et al. Exceptional Response to Radio-Target Therapy in a Case of Metastatic Nodular Melanoma. Ann Med Health Sci Res. 2021;11:1527-1531.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Aim: Melanoma is the most aggressive and dangerous of the skin cancers and its incidence has been increasing for several decades. This pathology is therefore has become a major health issue for Western populations. Yet if the primary melanoma is generally of good prognosis, once the metastatic stage reached, no effective therapeutic solution is currently available. Targeted therapies promising are still being developed such as BRAF inhibitors. Case Report: We present a case of metastatic nodular melanoma. A 43-year-old woman was admitted to our hospital exophthalmia with brain, pulmonary and adrenal metastasis. Conclusion: The incidence of melanoma continues to rapidly increase in the world. Detecting molecular markers aids diagnosis and identification of mutations BRAF, not only helps to diagnosis and prognostic, but also to guide treatmentand radiotherapy can potentiate the response by immuno stimulatory property.
Keywords
Melanoma; Immunotherapy; Response; BRAF inhibitors; Metastatic cancer
Introduction
Melanoma is the most aggressive and dangerous of the skin cancers and itsincidence has been increasing for several decades. This pathology is thereforehas become a major health issue for Western populations. Yet if the primary melanoma is generally of good prognosis, once the metastatic stage reached, no effective therapeutic solution is currently available. Targeted therapies promising are still being developed such as BRAF inhibitors. However, many cases of resistance to these new compounds have already been identified. Thechemoresistance therefore remains a central concern in the case of melanoma. The chemo resistance as well as tumor aggressiveness have recently been associated with the existence cell subpopulations characterized by the expression of membrane markerssuch as CD20, CD271, CD133 or ABCB5.
Melanoma is a tumor proliferation developed at the expense of melanocytes, cells located in the basal layer of the epidermis within keratinocytes whose main function is the synthesis of melanin and it represents 287,723 (1.6) new cases of skin cancers, but it is by far the most fatal being responsible for 60,712 (0.6) of deaths linked to skin cancers. [1,2]
Nodular melanomas represented 7%-10% of all melanomas at present and are the second most common subtype of malignant melanocytic neoplasm, next to superficial spreading melanoma. [3]
Cutaneous melanoma is a multifactorial disease which depends mainly on the interaction between skin type and 13T UV exposure (period and intensity) and on individual factors (ethnic origins, genetic factors, skin pigmentation, behavior). Histological examination confirms the melanocytic nature of the tumor and determines the histo-prognostic criteria: Degree of invasion (Clark classification) and tumor thickness (Bresslow index). Cutaneous melanoma is the most aggressive of skin cancers and its dangerousness comes from its great capacity to form metastases. [4] At present, prevention and early detection of the primary lesion remains the only means of achieving a cure. Until now, few solutions existed in the management of metastatic melanoma based essentially on so-called “palliative” chemotherapy. Currently, the development of molecular biology has made it possible to address the molecular mechanisms of tumor progression and to shed light on the genes involved in this evolution. [5] However, significant difficulties persist: There is a great variability in the expression of these genes not only from one patient to another, but also according to the stages of the disease, local or metastatic, moreover, some of the mutations found. During melanoma can also be present in benign lesions (nevus). [6] The genotypic variability associated with melanoma makes therapeutic targeting complex and currently constitutes a major challenge in terms of treatment.
Case Report
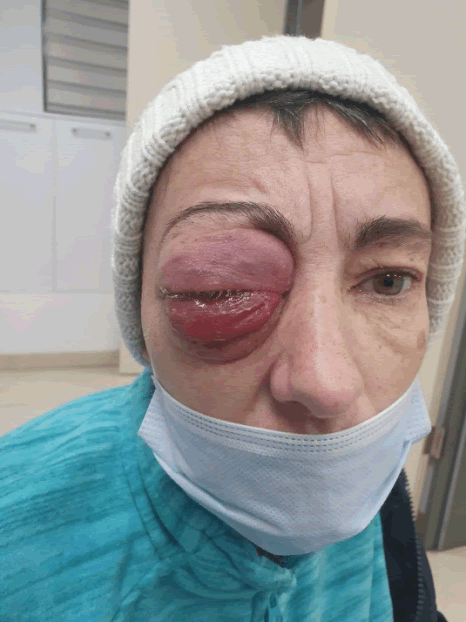
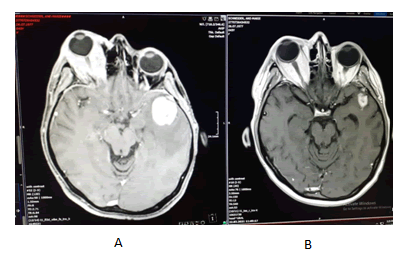
A 43-year-old woman was admitted to our hospital with brain metastasis and exophthalmia [Figure 1]. She complained of weight loss and lack of vision with the affected eye. She had no other co-morbidities or family history of malignancy.
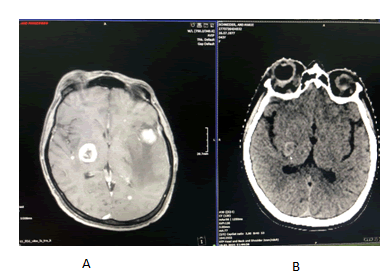
On the brain IRM was described signal distortion of the right orbital find with a native fringed appearance with intense gadolinophilia encompassing both the nerve and extrinsic muscles with exophthalmos. At the cerebral level there are countless sub and supratentorial secondary lesions with a hematoma component ranging from methemoglobin to hemosiderin. Subtentorial lesions are more numerous but smaller. With perilesional edema only in adults; they interest both cerebellar hemispheres right >left largest 13.5/11.4, upper vermis with 3 lesions on right. Up to 8.8 mm diameter; right bulbar macrolysis to the inferior cerebellar pendulum 13 mm/12.5 mm/11.5 mm diameter. Supratentorial lesions are more numerous bilaterally on the left with majority distribution to the peripheral giral and peripheral edema. They are small, bilateral occipital; lesions up to 6.5 mm bilateral superior parasagittal occipital; the frontal and parietal right lesions. Left fronto basal and right temporal internal have up to 4.5 mm located near the dura and 9 mm right frontal internal; at least 3 lesions left anterior parietal up to 7 mm in diameter and on convexity 3 lesions the largest 8 mm in diameter. There was also 2 macro lesions with necrotic-hemorrhagic character, one temporal left 28.5 mm ap/26 mm t/27.8 mm CC the other in the basal ganglia on the right side (lenticular) up to 20.7 mm cc/19 mm AP/18.5 mm T both with important peripheral digital form edema on the left infiltrating the nuclear capsules; mass effect of the temporal lesion on the left lateral ventricle slightly reduced in size without deviation from the midline with the imprint on the left cerebral pendulum [Figure 2].
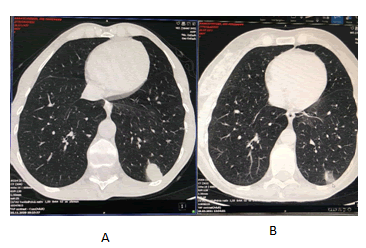
Thorough investigation, Computed Tomography (CT) of the torax, abdomen and pelvis showedat the left lower lobeof the lung, there is a tumour mass with axial diameters of about 24 mm/15 mm, in contact with the left postero-lateral thoracic wall, with irregular contour, inhomogeneous iodophilia and a tissue node most likely belonging to the left adrenal gland, with axial diameters of about 18 mm/15 mm, with suspicious characters. Adjacent, on the vascular path, also located at the LIS level, there are two other nodules with similar characters, one with a diameter of about 10 mm and the other of about 7 mm. A fine-needle aspiration of the supraclavicular lymph nodes was performed. The pathological reports described a nodular melanoma with a mitotic index of 30 mm2. The immunochemistry staining showed HMB 45 positive, Mel ARED positive, AE1/ AE3 and LCA negative. The genetic analysis reported a BRAF codon 600 mutation in exon 15.
We started Wall Brain Radiotherapy (WBRT) for brain metastasis but after 5 days the we had to stop due to the fact that the exophthalmiaworsened and the patient’s mask did not fit and if we cut it was no longer fixed to the radiotherapy table. The patient was changed to therapy with dabrafenib and trametinib. Combination therapy with BRAFi and MEKi was well tolerated.
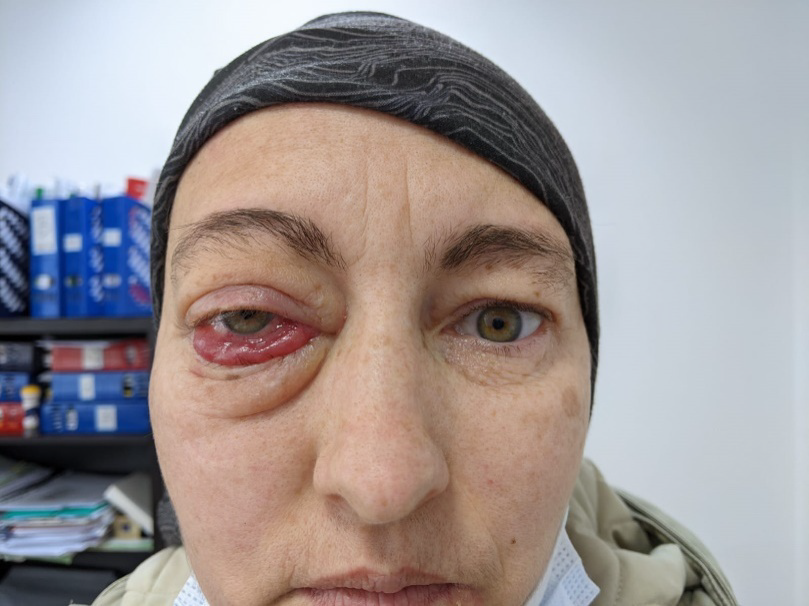
After one month the patients presented to the hospital with an important reduction of the exophthalmia and and with the improvement of the general condition [Figure 3].
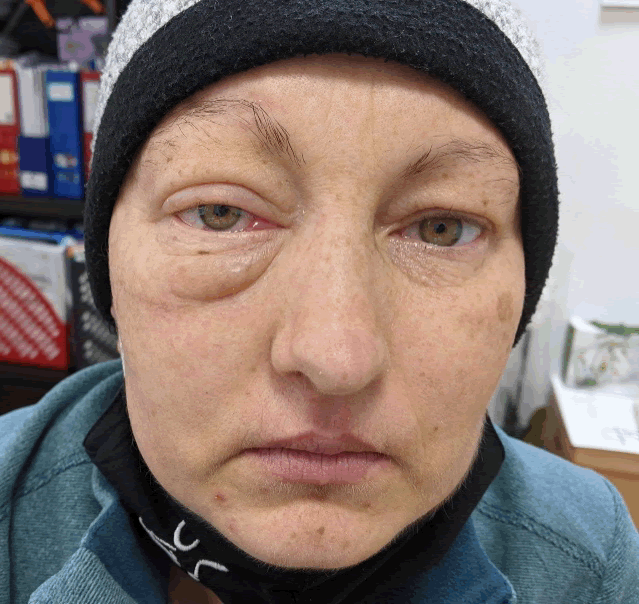
We continued the radiotherapy sessionsuntil completion. After three months of treatment the patient showed an amazing clinical evolution with the significant reduction of exophthalmia [Figure 4], recovery of vision and weight gain. The brain Magnetic Resonance Imaging (MRI) [Figure 5] and Computed Tomography (CT) scan of the torax, abdomen and pelvis showed [Figure 6] the reduction of the intra orbital tumour lesion, the size of lesions in the brain lung and adrenal had decreased markedly withno evidence a new lesions. No adverse reactions were observed during the period of treatment and with good blood tests.
Discussion
Melanoma is the most aggressive and dangerous of the skin cancers and its incidence has been increasing for several decades. The main risk factors is exposure to sunlight, which produces variations in DNA and its suppression of cellular apoptosis. [6] Nodular melanomas represented 7%-10% of all melanomas at present and are the second most common subtype. It has an orientation to lymphatic and haematic dissemination, and up to 4% of cases it is discovered by metastasis. [3]
Due to its aggressive nature and association with an increased mortality rate, a study was conducted that developed and validated an easy-to-use example nomogram for predicting 3-year and 5-year survival rates in nodular melanoma patients [7] and considerable efforts have been made in recent years to treat metastatic melanoma.
Immunotherapy is a novel approach and had great significance for the treatment of melanoma. The current strategy for treating melanoma with immunotherapy is to block the control points of the immune system with monoclonal antibodies called immune checkpoint inhibitors. These antibodies target inhibitory receptors present on the surface of lymphocytes (CTLA-4 or PD-1) and their initial action in the lymphoid organs (anti- CTLA-4) and/or directly in the tumour microenvironment (anti- PD-1). [8-10]
Currently we can use thisimmune checkpoint inhibitors in adjuvant and in metastatic cases and targeted therapy. [11,12] About 40% to 50% of melanomas are carriers of a BRAF gene mutation responsible for activating the channel MAP kinases. The most common BRAF mutation is V600E, which represents 80% of alterations in the gene. [13] Two BRAF selective inhibitors currently we can use in metastatic melanoma: Vemurafenib and Dabrafenib.
Although BRAF inhibitors represent a real therapeutic revolution in metastatic melanoma,the duration of the response appears limited in time, revealing the occurrence of multiple resistance mechanisms better and better described today. In order to reduce or delay this escapement, studies associating an anti-MEK and an anti-BRAF have been carried out. Three major phase trialsIII were recently published. They have showed the superiority of the association BRAF inhibitor and inhibitor of MEK versus BRAF inhibitor alone. [14-16]
Maintaining long-term response is difficult, with just 20% of patients in one study remaining progression free. [17,18] Ongoing studies investigating the addition of CDK, MAPK, or immune checkpoint inhibitors to combat treatment resistance. [19-21] They are not described in the literature and no studies have been performed that include patients with nodular melanoma treated with combined inhibition of Dabrafenib/Trametinib. The main goal of radiotherapy is to maximize the therapeutic margin by delivering a high dose of radiation to the tumor tissue while sparing as much of the surrounding tissue as possible. However, it has been shown that certain effects induced by RT on un-irradiated tumor cells can have a positive impact on clinical outcome. Tumor regression on non-irradiated as a result of such effects is called an abscopal effect. [22]
Since the first description in 1953 [23], the abscopal effect has been observed in different tumor entities. The mechanism has not been fully elucidated, but in summary, the most likely explanation is that it is caused by systemic immune activation due to tumor cell death in the irradiated target volume. [24]
Locally dead tumor cells and their released antigens generate an immune response, also known as in situ vaccination. Activated immune effector cells can then attack tumor cells far removed from the treated lesion. [25] Although well documented, the abscopal effect remained a phenomenon limited to individual case reports. With the advent of RT, the immune stimulatory property of RT returned to the forefront of the scene. [26] A marked increase in abscopic responses was observed in combination with ICIs; these can be observed in the case of malignant melanomas in a proportion of up to one-third, which may be clinically relevant. [27]
Based on the tumour immune cycle described above, the many possible mechanisms of immune modulation by RT can be classified. In the early stages, RT increases the presentation and diversity of tumour-specific antigens. Radiation-induced immunogenic cell death releases tumour antigens, tumour cell DNA, cytokines, and other “danger signals”. [22,24,28] Genetic damage caused by radiation increases the mutation load and results in the generation and release of neoantigens. Better antigen recognition stimulates dendritic cells, leading to increased activation of cytotoxic T cells as part of priming. [24]
Irradiation-altered immune microenvironment in and around the tumour cell also promotes the effectors phase steps regarding tumour cell destruction. [29] Modifications of the endothelium of the tumour’s blood vessels facilitate the adhesion of circulating effector immune cells and promote their migration into the tumour. A higher number of infiltrating cells is positively correlated with the clinical outcome in many tumours. [30-32] Increased expression of Major Histocompatibility Complex I (MHC I) on the surface of irradiated tumour cells increases the destruction of tumour-targeting cells by effector T cells. [33,34] In addition to immune stimulatory processes, RT can also inhibit the immune system, as is known from the irradiation of inflammatory diseases, where very small single doses are mainly applied. However, even these mechanisms can be used as an argument for a combination with IT, since, for example, up regulation of the PD-1/PD-L1 signaling pathway (to inhibit the immune response) provides a target for ICIs and can increase their effectiveness. [35]
In the case of malignant melanoma, RT has been widely proven and has become the standard of care in adjuvant and palliative treatment situations. According to a retrospective study, patients treated with a PD-1 inhibitor and palliative RT achieved significantly better response rates (65% vs. 33.3%) with comparable toxicity. [36] The combination of ipilimumab and RT resulted in a prolonged overall survival in addition to significantly higher response rates than those obtained with ipilimumab alone. [37] The combination has been prospectively evaluated as safe in Phase I data to date. [38-40]
However, the patient described in this case reportdiagnosed with BRAF-mutant metastatic nodular melanoma achieved a clinical benefit from this treatment.
Conclusion
The incidence of melanoma continues to rapidly increase in the world. Detecting molecular markers aids diagnosis and identification of mutations BRAF, not only helps to diagnosis and prognostic, but also to guide treatment. We present the first patient with a BRAF-mutant metastatic nodular melanoma who has been effectively treated with a combined use of BRAF/ MEK inhibitor and radiotherapy.
Conflicts of Interest
The Authors declare that they have no competing interests in relation to this study.
Author's Contributions
BH was responsible writing of the manuscript. AZ, XB, was responsible for reviewing and editing of the manuscript. AZ, BH, SM, XB and RA made substantial contributions to the conception or design of the work. RA were responsible for the critically reviewed the manuscript. All Authors read and approved the final manuscript.
REFERENCES
- MacKie RM. Malignant melanoma: Clinical variants and prognostic indicators. Clin Exp Dermatol. 2000;25:471-475.
- https://gco.iarc.fr/today/data/factsheets/cancers/16-Melanoma-of-skin-fact-sheet.pdf
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130.
- Bergenmar M, Ringborg U, Månsson BE, Brandberg Y. Nodular histo genetic type the most significant factor for thick melanoma: Implications for prevention. Melanoma Res. 1998;8:403-411.
- McClay EF, Mastrangelo MJ, Berd D, Bellet RE. Effective combination chemo/hormonal therapy for malignant melanoma: Experience with three consecutive trials. Int J Cancer. 1992;50:553-556.
- Heasley DD, Toda S, Mihm MC. Pathology of malignant melanoma. Surg Clin North Am. 1996;76:1223-1255.
- Yang J, Pan Z, Zhao F, Feng X, Liu Q, Li Y et al. A nomo gram for predicting survival in patients with nodular melanoma: A population-based study. Medicine. 2019;98:e16059.
- Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell. 2015;161:205-214.
- Mellman I, Coukos G, Dranoff. Cancer immunotherapy comes of age. Nature. 2011;480:480-489.
- Hodi FS, Chiarion SV, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab plus ipilimumab or nivolumab alone vs. ipilimumab alone in advanced melanoma (check mate 067): 4-year outcomes of a multicentre, randomised, phase 3 trials. Lancet Oncol. 2018;19:1480-1492.
- Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672-681.
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694-1703.
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Dabrafenib and trametinib vs. dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet. 2015;386:444-451.
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Grob JJ, Stroyakovskiy D, Karaszewska B, Hauschild A, Levchenko E, et al. Five-Year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019;381:626-636.
- Long GV, Weber JS, Infante JR, Kim KB, Daud A, Gonzalez R, et al. Overall survival and durable responses in patients with braf v600-mutant metastatic melanoma receiving dabrafenib combined with trametinib. J Clin Oncol. 2016;34:871-878.
- Amann VC, Ramelyte E, Thurneysen S, Pitocco R, Bentele-Jaberg N, Goldinger SM, et al. Developments in targeted therapy in melanoma. Eur J Surg Oncol. 2017;43:581-593.
- Pavlick AC, Fecher L, Ascierto PA, Sullivan RJ. Frontline therapy for BRAF-mutated metastatic melanoma: How do you choose, and is there one correct answer?. Am Soc Clin Oncol Educ. 2019;39:564-571.
- Martin CA, Cullinane C, Kirby L, Abuhammad S, Lelliott EJ, Waldeck K, et al. Palbociclib synergizes with BRAF and MEK inhibitors in treatment naïve melanoma but not after the development of BRAF inhibitor resistance. Int J Cancer. 2018;142:2139-2152.
- Chen DS, Mellman I. Oncology meets immunology: The cancer-Immunity cycle. Immunity. 2013;39:1-10.
- Mole RH. Whole body irradiation-radiobiology or medicine? Br J Radio. 1953;26:234-241.
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol. 2017;17:97-111.
- Derer A, Deloch L, Rubner Y, Fietkau R, Frey B, Gaipl US. Radio-immunotherapy-induced immunogenic cancer cells as basis for induction of systemic anti-tumor immune responses-pre-clinical evidence and ongoing clinical applications. Front Immunol. 2015;6:505.
- Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol. 2018;39:644-655.
- Chicas SR, Morales OI, Rodriguez AD, Lara JP. Combining radiotherapy and ipilimumab induces clinically relevant radiation-induced abscopal effects in metastatic melanoma patients: A systematic review. Clin Transl Radiat Oncol. 2018;9:5-11.
- Asna N, Livoff A, Batash R, Debbi R, Schaffer P, Rivkind T, et al. Radiation therapy and immunotherapy:A potential combination in cancer treatment. Curr Oncol. 2018;25:e454-460.
- Weichselbaum RR, Liang H, Deng L, Fu YX. Radiotherapy and immunotherapy: A beneficial liaison? Nat Rev Clin Oncol. 2017;14:365-379.
- Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38:1074-1084.
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31:860-867.
- Bacinschi XE, Zgura A, Safta I, Anghel R. Bio molecular factors represented by Bcl-2, p53, and tumor-infiltrating lymphocytes predict response for adjuvant anthracycline chemotherapy in patients with early triple-negative breast cancer. Cancer Manag Res. 2020;12:11965-11971.
- PopaVO, Bacinschi X. Dynamics of anxiety in cervical cancer patients undergoing radiotherapy: Preliminary results. J Psychosom Res. 2019;121:135.
- Galon J, Costes A, Sanchez CF, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964.
- Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francisca BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated anti-tumor immune responses via cross- presentation of tumor antigen. Cancer Immunol Res. 2016;25:289-313.
- Aboudaram A, Modesto A, Chaltiel L, Gomez RC, Boulinguez S, Sibaud V, et al. Concurrent radiotherapy for patients with metastatic melanoma and receiving anti-programmed-death 1 therapy: A safe and effective combination. Melanoma Res. 2017;27:485-491.
- Koller KM, Mackley HB, Liu J, Wagner H, Talamo G, Schell TD, et al. Improved survival and complete response rates in patients with advanced melanoma treated with concurrent ipilimumab and radiotherapy versus ipilimumab alone. Cancer Biol Ther. 2017;18:36-42.
- Twyman SVC, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373-377.
- Hiniker SM, Reddy SA, Maecker HT, Subrahmanyam PB, Rosenberg HY, Swetter SM, et al. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int J Radiat Oncol Biol Phys. 2016;96:578-588.
- Rodica MA, Minea LN, Bacinschi H, Isacu I, Tarlea A. Concomitant radical non-platinum radio chemotherapy for elderly patients with invasive bladder cancer. J Clin Oncol. 2009;27:e16135-e16135.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.