Expression of Inducible Nitric Oxide Synthase in the Epithelial Linings of Odontogenic Keratocyst, Dentigerous Cyst and Radicular Cyst: A Pathological Insight
- *Corresponding Author:
- Dr. P. Swetha
Department of Oral and Maxillofacial Pathology, Vishnu Dental College, Bhimavaram, Andhra Pradesh, India.
E-mail: drswe82@yahoo.co.in
Abstract
Background: The present study is aimed at analyzing the expression of inducible nitric oxide synthase (iNOS) in the epithelial lining of odontogenic keratocyst (OKC), dentigerous cyst (DC), radicular cyst (RC) in order to understand the possible role of iNOS with special reference to its neoplastic nature and local aggressive of cysts. Aim: The primary aim of the following study is to analyze the immunohistochemical expression of iNOS and secondary aim is to compare the iNOS expression, pattern and intensity of staining among the epithelial linings of OKC, DC and RC. Materials and Methods: iNOS in the epithelial lining cells were analyzing in 10 OKC’s, 10 DC’s and 10 RC’s using immunohistochemistry. The percentage of positive cells was assessed and presented as mean ± standard deviation. The correlation with respect to the intensity and percentage of staining within the epithelial linings of OKCs, DCs and RCs was carried out using (analysis of variance and Student’s t‑test) Chi‑square test. Results: Staining intensity of iNOS portion was seen in the entire thickness of the epithelial linings of OKC, whereas in DC’s only one case had entire thickness of the epithelial lining staining and in RC’s none of the cases showed entire thickness of staining. On comparing the staining intensity of iNOS between OKC, DC and RC groups, using Chi‑square test, there was a statistically significant difference between these groups (P < 0.01). On analyzing the immuno‑reactivity of iNOS in OKC, DC and RC there was a positive variable expression iNOS between the cysts. Conclusion: iNOS was over expressed in OKCs when compared with DC and RC suggesting that iNOS may contribute to the aggressive behavior of OKC. This is yet another evidence to support that OKC is the neoplasm.
Keywords
Dentigerous cyst, Immunohistochemistry, Inducible nitric oxide synthase, Odontogenic keratocyst, Radicular cyst
Introduction
Odontogenic cysts are common lesions of oral and maxillofacial region occurring within the jaws with an origin from the tissue of tooth formation. Odontogenic keratocysts (OKCs), dentigerous cysts (DCs) and radicular cysts (RCs) are the three most common odontogenic cysts.[1]
Despite considerable efforts, little is known about the biological processes governing the initiation and growth of these cysts, especially OKC which is of particular interest owing to its aggressive clinical behavior, recurrence and increase in its neoplastic potential when compared with DC and RC.[2]
Recent studies have shown high nitric oxide (NO) production in the cysts may participate in bone resorption and cystic enlargement, due to synthesis of matrix metalloproteinases which can be activated by NO.[3,4] NO is synthesized by a complex family of enzymes called NO synthases (NOS).
NO has been called a “double-edged sword”[5] with beneficial antiviral, antiparasital, microbicidal, immunomodulatory and anti-tumoral effect[6] and deleterious effects such as inhibition of enzyme functions, alteration of deoxyribonucleic acid, induction of lipid peroxidation, mutation of tumor suppressor genes, cytotoxicity, inhibition of mitochondrial respiration, depletion of antioxidant stores and hypoxia induced angiogenesis in cancer depending on the amounts and conditions under which it is produced.[7] In neoplastic tissue, NO is implicated as having a positive role in permitting tumor growth, including mutagenicity, angiogenesis and metastasis.
NOS exists as three isoforms; the calcium dependent endothelial NOS (eNOS), neuronal NOS (nNOS) and calcium independent inducible NOS (iNOS). From a functional perspective, it is important to recognize that induction of the high-output iNOS usually occurs in an oxidative environment and thus leads to react with superoxide leading to peroxynitrite formation and cell toxicity. Cells require a complex cytokine combination including interferon gamma, interleukin (IL)-1β and tumor necrosis factor-alpha for iNOS induction.[8]
Expression of iNOS has been studied in various cysts and some of the reports have emphasized the significance of iNOS expression as an indicator of the malignant potential of cysts. The purpose of the current study is to assess the immunohistochemistry expression of iNOS, in an attempt to justify the neoplastic nature and the biological behavior of the cysts from the standpoint of the difference in staining pattern in the epithelial linings of OKC in comparison with that of DC and RC.
Materials and Methods
The sample size in the present study consisted of 10 OKCs, 10 RCs, 10 DCs form the files of the Department of Oral Pathology, Rajah Muthiah Dental College and Hospital (RMDCH), Annamalai University. All the tissues obtained for the study were approved by the ethical committee from the institute.
All the specimens were fixed in 10% buffered formalin and embedded in paraffin wax, section of 3 μm thickness were sliced from the tissue blocks and stained with H and E and further examined morphologically to confirm the diagnosis and other sections were mounted on glass slides coated by APES (Sigma-Aldrich Chemical Co., USA) and processed for subsequent immunohistochemical study.
Blocking of endogenous peroxidase activity with 3% H2O2 for 10 min followed by treating with power block for another 10 min to avoid cross reactions. Antigen retrieval was done by immersing the sections in Tris ethylene-diamine-tetraacetic acid buffer solution, which in turn was kept in a pressure cooker containing water and was bought to full pressure by closing the lid and kept for 10 min duration. The cooker was allowed to cool down to room temperature before removal of slides, the sections were covered completely with optimally diluted rabbit polyclonal antibody against iNOS (Santa Cruz, Genetix) in 1:50 dilutions in phosphate buffered saline (PBS) for 1 hr.
After gentle washing in the PBS buffer water for 5 min, slides were treated with poly horse radish peroxidase for 30 min, followed by immune staining with 3,3’-diamino benzidine tetrahydrochloride for 5 min and counterstained with Mayer’s hematoxylin and sections were dehydrated in alcohol for 10 min and air dried thoroughly and mounted using dibutyl phthalate xylene.
The immune stained slides were observed for positivity under ×10/×40 magnifications and recorded with a high quality photo micrograph. The staining was scored by evaluating both the percentage and intensity of staining in representative regions of each specimen in OKC, DC, RC. The region of staining was scored as follows: 0, no staining of cells in any microscopic field; 1+, <25% of cells stains positive; 2+, between 25% and 50% cells staining positive; 3+, between 50% and 75% cells staining positive; 4+, >75% cells positive.
Statistical analysis
Statistical analysis was performed using Statistical Package for Social Science software, IBM and the level of significance of 0.05 was employed in all the statistical comparisons. Quantitative data were recorded as mean (± standard deviation). Statistical significance for the expression of iNOS in the OKC, DC, RC were analyzed using two-way analysis of variance (ANOVA) and paired Student’s ‘t’ test. Chi-square test was used to assess the staining intensity of the same.
Results
The present study carried out on 30 archival tissues available in the Department of Oral and Maxillofacial Pathology, RMDCH, Annamalai University. The samples of the study compose of three groups namely OKC, DC and RC. Under each group 10 specimens were included.
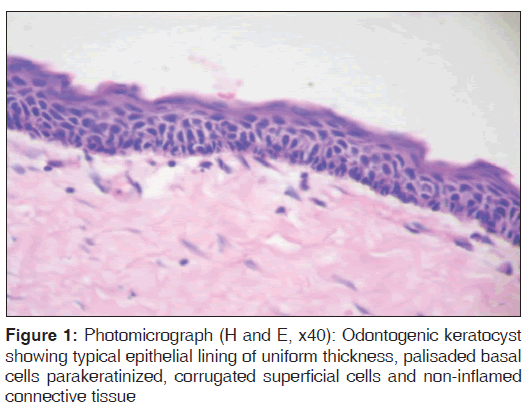
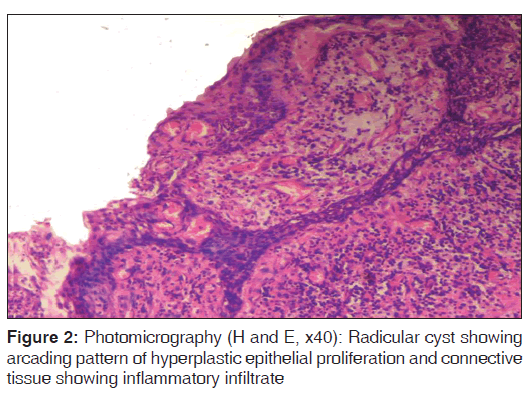
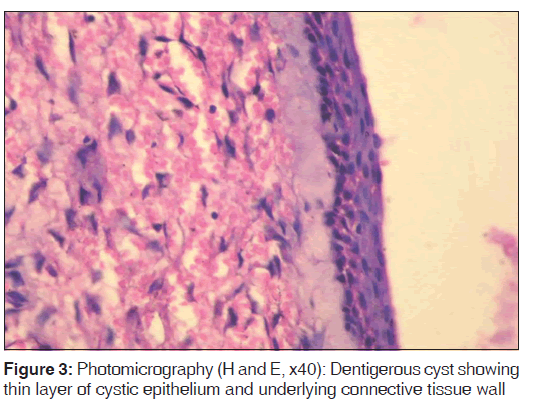
For each specimen, one slide was stained with eosin and hematoxylin to confirm the diagnosis and another was used for immunohistochemical analysis [Figures 1-3].
On analyzing the immunoreactivity of iNOS in OKC, DC and RC there was a positive variable expression of iNOS between the cysts.
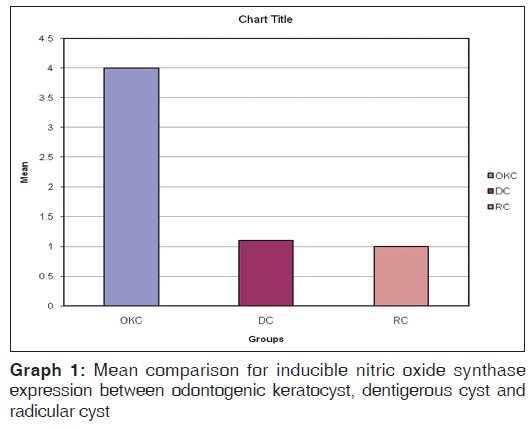
ANOVA for iNOS expression between the OKC, DC and RC were statistically significant with P < 0.01 [Table 1, Graph 1].
| Groups | N | Mean (SD) | Standard error | F value | P value |
|---|---|---|---|---|---|
| OKC | 10 | 4.00 (2.62) | 0.83 | 8.44 | <0.01 |
| DC | 10 | 1.10 (1.52) | 0.48 | ||
| RC | 10 | 1.00 (1.05) | 0.33 | ||
| Total | 30 | 2.03 (2.28) | 0.42 |
ANOVA: Analysis of variance, iNOS: Inducible nitric oxide synthase, OKC: Odontogenic keratocyst, DC: Dentigerous cyst, RC: Radicular cyst
Table 1: ANOVA for iNOS expression between OKC, DC and RC
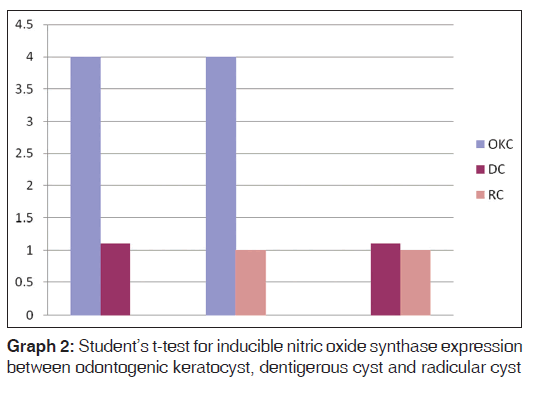
There was statistically significant difference in the iNOS expressions within the OKC and DC (P < 0.01), OKC and RC (P < 0.01) where as there was no statistically significant difference between DC and RC (P < 0.01) [Table 2, Graph 2].
| Groups | n | Mean (SD) | t value | P value |
|---|---|---|---|---|
| OKC | 10 | 4.00 (2.62) | 3.02 | <0.01 |
| DC | 10 | 1.10 (1.52) | ||
| OKC | 10 | 4.00 (2.62) | 3.35 | <0.01 |
| RC | 10 | 1.00 (1.05) | ||
| DC | 10 | 1.10 (1.52) | 0.17 | 0.86 |
| RC | 10 | 1.00 (1.05) |
iNOS: Inducible nitric oxide synthase, OKC: Odontogenic keratocyst, DC: Dentigerous cyst, RC: Radicular cyst, SD: Standard deviation
Table 2: Student t test for iNOS expression between OKC, DC and RC
Staining intensity of iNOS in each case was visually evaluated and assigned to one of the following categories: No staining, mild staining, moderate staining and strong intensity.
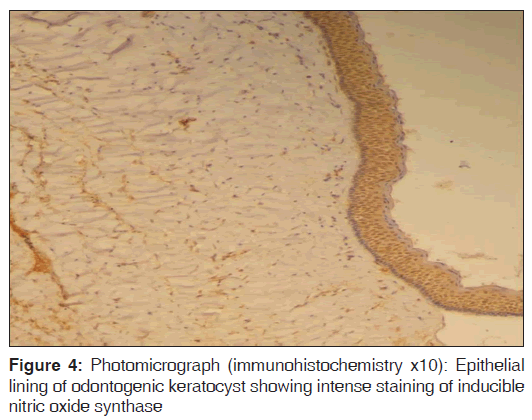
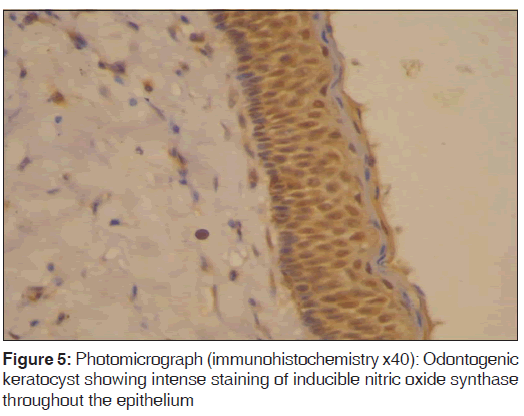
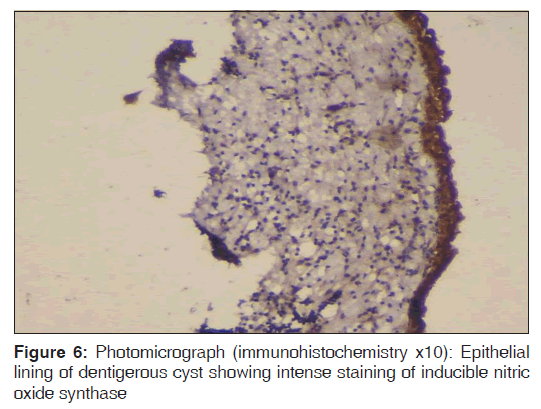
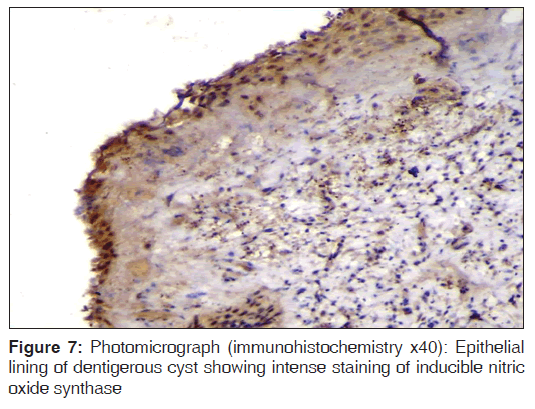
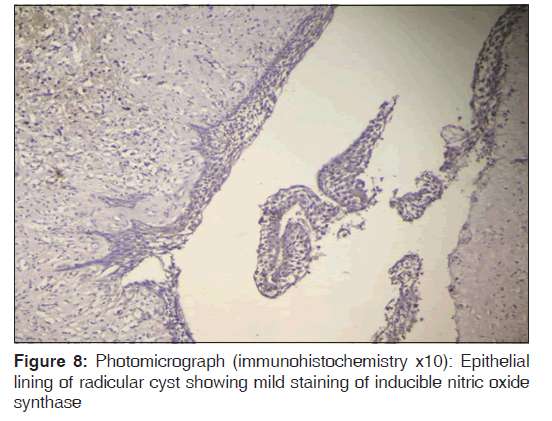
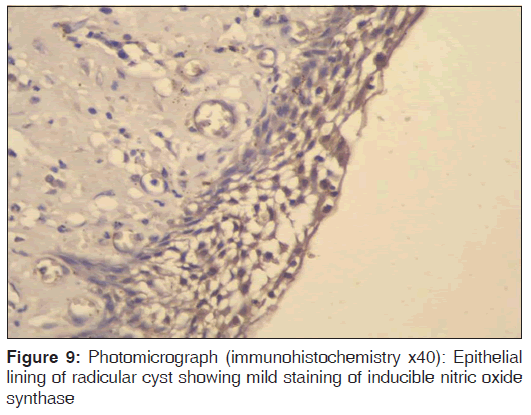
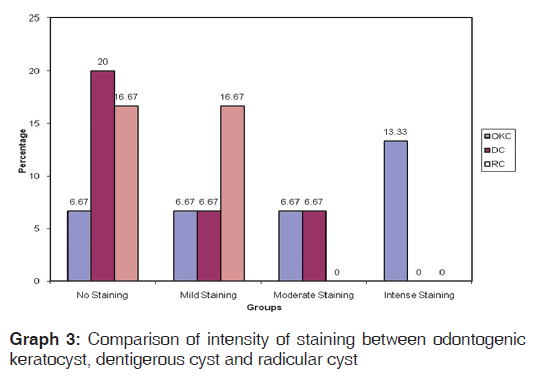
In the present study, we observed both the cytoplasmic and nuclear staining throughout the epithelial lining cells of OKC, DC and RC shows immunohistochemical positivity of iNOS protein was seen in the entire thickness of the epithelial linings of 10 OKCs in which four cases were intensely stained (13.3%) [Figures 4 and 5], two cases were moderately stained (6.7%), two cases were mildly stained (6.7%) and two cases showed no staining. Whereas in DCs, out of 10 cases, one case (3.3%) was intensely stained [Figures 6 and 7], two cases were moderately stained (6.7%), three cases were mildly stained (10%) and four cases showed no staining. Unlike OKCs and DCs, in RCs zero cases were intensely stained, zero cases were moderately stained, four cases were mildly stained (13.3%) [Figures 8 and 9] and six cases showed no staining (20%) [Table 3].
| Groups | No staining (%) | Mild staining (%) | Moderate staining (%) | Strong intense staining (%) | Total (%) | |
|---|---|---|---|---|---|---|
| OKC | 2 | (6.7) | 2 (6.7) | 2 (6.7) | 4 (13.3) | 10 (33.3) |
| DC | 4 (13.3) | 3 (10) | 2 (6.7) | 1 (3.3) | 10 (33.3) | |
| RC | 6 (20) | 4 (13.3) | 0 | 0 | 10 (33.3) | |
| Total | 13 | (43.3) | 9 (30.0) | 4 (13.3) | 4 (13.3) | 30 (100) |
iNOS: Inducible nitric oxide synthase, OKC: Odontogenic keratocyst, DC: Dentigerous cyst, RC: Radicular cyst
Table 3: Assessment of staining intensity of iNOS in OKC, DC and RC
On comparing the staining intensity of iNOS between OKC, DC and RC groups, by using Chi-square test, there was a statistically significant difference between these groups (P < 0.01) [Table 4, Graph 3].
| Calculated Chi square value | Degrees of freedom | Level of significance |
|---|---|---|
| 12.67 | 6 | <0.01 |
iNOS: Inducible nitric oxide synthase, OKC: Odontogenic keratocyst, DC: Dentigerous cyst, RC: Radicular cyst
Table 4: Assessment of staining intensity of iNOS in OKC, DC and RC
Although there was a variation in the staining intensity of each lesion, most OKCs showed high intensity of staining while DCs and RCs. Despite the expression of iNOS in RCs, the percentage of iNOS positive cells in RCs was lower than those of OKCs and DCs.
Discussion
By definition, cysts are pathological cavities lined with or without epithelium, containing fluid, semi-fluid or gaseous contents. Odontogenic cysts have their origin from the glands of serres (rests of the dental lamina), the rests of malassez (rests of the root sheath of hertwig) and the reduced enamel epithelium (remnants of the enamel organ after dental crown formation). In case of OKC it has been proposed that the lining may also be derived from mucosal basal cells.[9] Based on etiology of these lesions, it has been traditionally classified into two different groups: developmental (DC, OKC, etc.) and inflammatory (RC) cysts. In terms of their incidence, RC is the commonest followed by DC and OKC.[10]
Most of the odontogenic cysts have characteristic epithelial lining and they differ in their biological behavior. Although the majority of epithelial cysts are thought to grow passively driven by hydrostatic pressure inside the lumen, some cysts show active cellular proliferation.[11] In case of OKC, they commonly show active epithelial growth which has prompted the belief that they should perhaps be regarded as neoplasms rather than cysts. This seems to be supported by the observation that the epithelial cells in the lining of these lesions possess genetic abnormalities in specific tumor suppressor genes.[10].
In an article in 1967, Toller suggested that the OKC may be best regarded as a benign neoplasm rather than a conventional cyst based on its clinical behavior. In comparison to other cysts of the jaws, OKC shows characteristic clinical features, including potentially aggressive behavior and high recurrence rate. OKC is now designated by the World Health Organization as a neoplasm, keratocystic odontogenic tumor (KCOT). Several factors form the basis of this decision: locally destructive and highly recurrent nature, budding of the basal layer in to the connective tissue, presence of mitotic figures in suprabasal layer, allelic loss of 9q22 by two hit mechanism.[12]
The unique nature of OKC and the recent shift in the concept of OKC as a ‘tumor’ made us evaluate yet another factor, iNOS, an enzyme which has been implicated in the tumorogenesis of various neoplasms.
The role of NO generated by the iNOS is very complex. However, induced at the wrong place or at the wrong time, iNOS has detrimental consequences and seems to be involved in the pathophysiology of different human diseases. The pathways regulating iNOS expression seem to vary in different cells or different species.[13]
There have been few published reports in this area and only few investigated the iNOS expression in odontogenic cysts. It has been demonstrated that the epithelial lining cells of OKC, DC and RC can produce their own cytokines such as IL-1α and IL-6. These cytokines may activate iNOS expression of these epithelial cells in an autocrine fashion.[1]
As the results of our study were evaluated, the investigation focused on whether there was an obvious difference in terms of immunoreactivity of iNOS in the epithelial lining cells among OKC, DC and RC. In the present study, we examined 30 odontogenic cysts (10 OKC, 10 DC and RC) of which 17 cysts (57%) showed immunoreactive positive cells of varying degrees in their epithelial linings. This could be attributed to the (a) non-inflamed OKC epithelial lining and (b) fragile epithelial lining of OKC with 2-3 layers of thickness in the present study. Another reason for the decreased iNOS expression could be less number of study sample material. This was in contrast to the earlier study by Poomsawat et al.[1] in which out of 60 odontogenic cysts (20 OKC, 20 DC and 20 RC), only 80% expressed 100% immuno-reactivity whereas, 20% expressed more than 80% immuno-reactivity to iNOS in the lining epithelium.
When the scores were analyzed using Student’s t-test between the different study groups, there was a statistically significant difference between OKC and DC and also between OKC and RC. However, there was no statistical significance when DC and RC were compared. The results clearly showed that the iNOS expression is more in OKC when compared with the other two cysts and it could be comparable to the iNOS expression levels studied in various tumors.
Many authors have suggested that the lining of OKC has high proliferative capacity and this proliferation could be due to increased mitotic index.[14] For example, the study by Li et al.[15] on immunohistochemical expression of proliferating cell nuclear antigen (PCNA), a cell proliferative marker in these cysts showed that OKC had much higher PCNA index when compared with DC and RC, supporting the notion that the proliferation in OKC lining is much higher. The increased proliferative capacity of OKC epithelium is also blamed for its biologically aggressive behavior.
In oral squamous cell carcinoma, the iNOS expression may be positively associated with p53 mutation, suggesting that iNOS could cause the mutation of p53 gene, which might further lead to the progression of tumor.[16] Earlier data on p53 mutation and thus the immuno-expression in OKC when compared with other odontogenic cysts suggest that a p53 mutation has a clear role in the development and progression of OKC. This fact is also be made as a supporting evidence to claim OKC as a neoplasm in the past.[1]
Consistently higher expression of iNOS obtained in our study and the previous evidences relating the role of iNOS and the evasion of p53 dependent apoptosis clearly indicate that iNOS too has a definite role in the development and progression of OKC.
Yet another role of iNOS in many of the tumors is its pivotal role in the tumor induced angiogenesis, a process central to tumor growth, progression and metastasis. iNOS dependent NO controls angiogenesis by modulating the expression of vascular endothelial growth factor (VEGF), the prime growth factor involved in endothelial cell proliferation and sprouting of blood vessels. The relationship among iNOS, VEGF and vascular density in tumors is established in many recently reported studies.[17]
Apart from tumorogenesis, the role of iNOS in inflammation is also commendable as iNOS is also secreted by endothelial cells and most of the inflammatory cells including macrophages. It has been shown that various inflammatory cytokines can help in the stimulation of iNOS induction which can modulate many aspects of cellular growth, differentiation and activation and might be associated with initiation of inflammation.[18] In our study, we also found iNOS expression in the inflammatory cells of most of the RC and some of the DC, especially in the infected areas which justifies its role in the zone of inflammation as a modulator.
An increased expression of OKC to the extent of neoplasm in our study and the already established findings of its role in the cell proliferation, angiogenesis and production of proteolytic enzymes, suggest that there is a define role of iNOS in the development and progression of OKC. It also justifies the differing biologic behavior of the OKC compared with other odontogenic cysts. This study establishes yet another evidence to support the notion of OKC being a neoplasm (KCOT). Yet another evidence to support the notion of OKC being a neoplasm (KCOT).
Conclusion
In the present study, the results clearly showed that there is increased expression of iNOS in OKC when compared with DC and RC, suggesting that iNOS may contribute to the aggressive behavior of OKC. It also justifies that iNOS plays an important role in the pathology of both inflammatory and developmental lesions. This is yet another evidence to support that OKC is a neoplasm.
Despite considerable effort, however, little is known about the cellular and molecular mechanism underlying the development and maintenance of this cyst type. The exact role of iNOS in the neoplastic potential of OKC is still rudimentary. It is therefore, crucial to identify genes and/or their protein products that may have a role in the pathogenesis of this clinically important lesion.
The importance of variations among these three cysts requires further investigation. Further study, with more number of samples, are encouraged to understand the precise role of iNOS activity in these cysts.
Acknowledgment
I would like to thank Dr. Bina Kashyap, Associate Professor, Oral and Maxillofacial Pathology for her continuous support.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- P o o m s a w a t S , P u n y a s i n g h J , V e j c h a p i p a t P . Immuno-histochemical expression of p53 protein and iNOS in odontogenic cysts. J Med Assoc Thai 2009;92:952-60.
- Li TJ, Kitano M. Epithelial cell proliferation in the odontogenic keratocyst: A review. Cell Prolif Odontogenic Keratocyst 1997;17:27-40.
- Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, et al. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: A novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys 1997;342:261-74.
- Rodini CO, Batista AC, Dionísio TJ, Santos CF, Cunha FQ, Lara VS. Morphologic evaluation and expression of matrix metalloproteinases-2 and 9 and nitric oxide during experimental periodontal disease in rat. J Mol Histol 2008;39:275-82.
- Brennan PA, Conroy B, Spedding AV. Expression of inducible nitric oxide synthase and p53 in oral epithelial dysplasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2000;90:624-9.
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: A review. Crit Rev Oral Biol Med 1993;4:197-250.
- Browne RM, Smith AJ. Pathogenesis of odontogenic cysts I. In: Browne RM, editor. Investigative Pathology of the Odontogenic Cysts. Boca Raton: CRC Press; 1999. p. 88-109.
- Lau YT, Ma WC. Nitric oxide inhibits migration of cultured endothelial cells. Biochem Biophys Res Commun 1996;221:670-4.
- Agaram.N, Bobby MC, Leon B, Deren L, Dalal A, Patrica S, Sydney F, Jennifer LM., Neoplastic status of odontogenic keratocysts. Arch Pathol Lab Med 2004;128:313-17.
- Giardina C, Caniglia DM, Lettini T, Valente T, Poliseno G, Tantimonaco L, et al. Morphometric discrimination between syndromic (Gorlin) and non-syndromic keratocysts. Anal Quant Cytol Histol 2001;23:373-80.
- Landini G. Quantitative analysis of the epithelial lining architecture in radicular cysts and odontogenic keratocysts. Head Face Med 2006;2:4.
- Madras J, Lapointe H. Keratocystic odontogenic tumour: Reclassification of the odontogenic keratocyst from cyst to tumour. J Can Dent Assoc 2008;74:165-165h.
- Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol 2004;500:255-66.
- Main DM. The enlargement of epithelial jaw cysts. Odontol Revy 1970;21:29-49.
- Li TJ, Browne RM, Matthews JB. Epithelial cell proliferation in odontogenic keratocysts: A comparative immunocytochemical study of Ki67 in simple, recurrent and basal cell naevus syndrome (BCNS)-associated lesions. J Oral Pathol Med 1995;24:221-6.
- Calmels S, Hainaut P, Ohshima H. Nitric oxide induces conformational and functional modifications of wild-type p53 tumor suppressor protein. Cancer Res 1997;57:3365-9.
- Jablonska E, Puzewska W, Grabowska Z, Jablonski J, Talarek L. VEGF, IL-18 and NO production by neutrophils and their serum levels in patients with oral cavity cancer. Cytokine 2005;30:93-9.
- Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.