Factors Associated with Prevalent Tuberculosis Among Patients Receiving Highly Active Antiretroviral Therapy in a Nigerian Tertiary Hospital
- *Corresponding Author:
- Dr. Iroezindu MO
Department of Medicine, College of Medicine, University of Nigeria, Enugu Campus, Enugu, Nigeria
E-mail: mikezindu@yahoo.com
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Abstract
Background: Tuberculosis (TB) causes significant morbidity/mortality among human immunodeficiency virus‑infected individuals in Africa. Reducing TB burden in the era of highly active antiretroviral therapy (HAART) is a public health priority. Aim: We determined the factors associated with prevalent TB among patients receiving HAART. Subjects and Methods: We conducted a cross‑sectional study of adult patients who had received HAART for ≥12 weeks in a Nigerian tertiary hospital. Patients whose TB diagnosis predated HAART were excluded from the study. Pre‑HAART data were collected from the clinic records, whereas post‑HAART data were obtained through medical history, physical examination, and laboratory investigations. Standard TB screening/diagnostic algorithms as applicable in Nigeria were used. Logistic regression analysis was used to determine factors independently associated with prevalent TB. Results: about 65.8% (222/339) were women. The mean age was 41.1 (10.0) years and 23.6% (73/339) had past history of TB. The prevalence of active TB was 7.7% (26/339). Among these patients, 42.3% (11/26) had pulmonary TB, 34.6% (9/26) had disseminated TB, whereas 23.1% (6/26) had only extra‑pulmonary disease. Only 45% (9/20) of patients with pulmonary involvement had positive sputum smear. Factors independently associated with prevalent TB were lower social class (adjusted odds ratio [aOR]: 31.7; 95% confidence interval [CI]: 1.1–1417.3), HAART non‑adherence (aOR125.5; 95% CI: 9.6–1636.3), baseline CD4 <200cells/μl (aOR31.0; 95%CI: 1.6–590.6), previous TB (aOR13.8; 95% CI: 2.0–94.1), and current hemoglobin <10 g/dl (aOR10.3; 95% CI: 1.1–99.2). Conclusion: Factors associated with prevalent TB were a lower social class, HAART non‑adherence, severe immunosuppression before HAART initiation, previous TB, and anemia post‑HAART. TB case finding should be intensified in these high‑risk groups.
Keywords
Factors, Highly active antiretroviral therapy, Human immunodeficiency virus, Prevalent, Tuberculosis
Introduction
Tuberculosis (TB) is the leading cause of morbidity and mortality among people living with human immunodeficiency virus (PLHIV) in developing countries.[1] HIV infection is the most important risk factor for TB and also modifies its clinical presentation.[2] The World Health Organization (WHO) estimates that about one-third of the 34 million HIV-infected people globally are infected with latent TB.[3] Individuals with TB-HIV co-infection are 21-34 times more likely to develop active TB disease than HIV-negative individuals.[3]
The use of highly active antiretroviral therapy (HAART) has led to a significant reduction in the burden of TB, mainly in countries with a low to medium TB burden.[4-6] However, TB remains a major public health problem in sub-Saharan Africa even in the era of HAART. In 2011, it was reported that 79% of the estimated 1.1million HIV-positive new TB cases globally were resident in sub-Saharan Africa.[3] Although the potential benefits of HAART on the burden of TB in sub-Saharan Africa is partly obviated by the unsatisfactory access to HIV treatment in the region, active TB in individuals who are receiving HAART occurs both in Africa and other settings with more established antiretroviral treatment programs.[7-10] Low immune status, especially CD4 count below 200 cells/µl remains a consistent risk factor for the development of active TB in patients receiving HAART in various populations.[8,10-12]
While the contribution of previous TB, poor nutritional status, and demographic factors appear to be prominent in a number of settings in sub-Saharan Africa,[10,11,13] these factors were not strongly associated with the occurrence of active TB in a report from South Africa.[8]
Nigeria ranks tenth among the 22 high TB burden nations and has the second highest number of PLHIV globally.[3,14] The prevalence of HIV among patients with TB in Nigeria increased from 2.2% in 1991 to 19.1% in 2001 and 25% in 2010 which suggests that the TB situation is strongly HIV-driven.[15] Unfortunately, only one-third of the proportion of HIV/TB co-infected patients in Nigeria received HAART in 2010.[15] In addition, TB prevention and control strategies in PLHIV in Nigeria are suboptimal considering that only 57% of the registered HIV-infected persons were screened for TB in 2010.[15] Inaddition, less than 1% of PLHIV among whom active TB was excluded received isoniazid preventive therapy in 2009.[16] So far, the risk factors for TB in patients receiving HAART in Nigeria remain poorly characterized. We therefore sought to determine the factors associated with prevalent TB among patients receiving HAART in a Nigerian tertiary hospital with a view to identifying potential recommendations to improve TB prevention and control in HIV-infected populations in the era of HAART.
Subjects and Methods
Study design
This was a cross-sectional study among HIV-infected patients receiving HAART conducted from April 1 to September 30, 2012.
Study setting/participants
The study was carried out at the Federal Medical Centre Owerri (FMCO), Imo state, South east Nigeria. The FMCO offers tertiary hospital services to the approximately 3.9 million people in the state including urban and rural settings,[17] as well as other neighboring states such as Abia, Anambra, and Rivers. Imo State has an estimated prevalence of HIV infection of 4.6%.[14] At the time of the study, the HIV clinic of FMCO otherwise known as heart-to-heart clinic provided services for about 5000 patients out of whom approximately 2000 adults were receiving HAART free-of-charge. At that time, the main eligibility criteria for HAART initiation at the clinic were baseline CD4cell count <200cells/µl and WHO clinical stages III or IV. As the clinic protocol, newly diagnosed HIV-infected patients are routinely screened for TB based on symptoms and chest radiographic features before initiation of HAART. In the course of follow-up during HAART, patients may have repeat laboratory investigations (usually chest radiography and sputum acid fast Bacilli [AFB] test) at any time they report symptoms suggestive of TB.
The study population comprised adult HIV-infected patients 15 years and older who had received HAART at the center for 12 weeks or more. In Nigeria, 15 years is the minimum age for enrollment into adult HIV treatment programs. Their HIV status was confirmed by Western blot assay. Patients who had received HAART for <12 weeks and those with TB diagnosed before commencement of HAART were excluded from the study. Furthermore, excluded were patients whose TB symptoms were poorly documented as well as those with missing results including sputum AFB, chest radiograph, and CD4 count.
Sample size and sampling procedure
This study was part of a larger study that primarily investigated the burden of opportunistic infections (OIs). In the larger study,[18] a minimum sample size of 322 patients was estimated at a 95% confidence interval based on a prevalence of OIs of 47.6% in a previous study in South Africa[19] using a population of approximately 2000 HIV-infected adults on HAART at the FMCO. To address the potential of having missing data, we collected an additional 10% of the initial estimated sample size for a total size of 354.
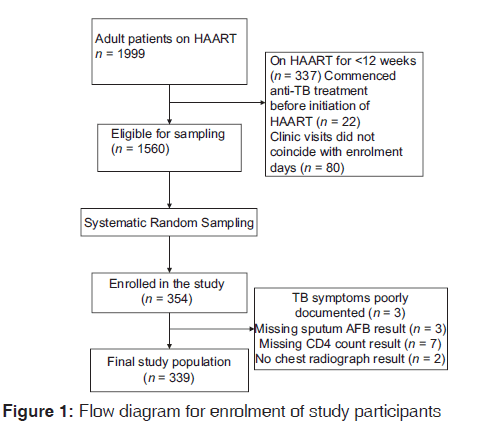
To ensure validity, a random sampling technique was adopted to minimize bias. After applying the exclusion criteria, a sampling frame was generated which comprised 1560 HAART-experienced HIV-infected patients. On each day of data collection, individuals in the sampling frame attending the clinic that day were identified. From the subset of HAART-experienced patients attending the clinic on each day, a random starting point was first chosen and subsequently every third person was selected. This was continued until the required sample size of 354 was attained. The flow diagram for patient enrollment is shown in Figure 1.
Data collection process
The data collection tool was an interviewer-administered questionnaire. To ensure reliability, a pilot testing of the questionnaire was carried out using 30 HIV-infected patients on HAART and pertinent minor modifications made before the commencement of data collection. The participants of the pilot study were excluded from the final study.
We collected demographic information such as age, gender, location of residence (rural or urban), and social class. We also obtained information on health behavior such as current alcohol use (yes or no) and cigarette smoking (ever smoked or never smoked). Data were also collected on pre-HAART (baseline) medical history, date of HIV diagnosis, WHO clinical stage at the time of diagnosis, baseline CD4 count, baseline hemoglobin, and date of HAART initiation.
Post-HAART data included medical history with emphasis on TB symptoms, physical examination, HAART adherence, current CD4 count, current hemoglobin, and investigations for TB where applicable. For the purpose of this study, the patients first underwent TB screening using a validated algorithm for HIV-infected patients.[20] A TB diagnostic algorithm was subsequently used to evaluate patients with a positive TB screening response (i.e., those who reported having at least one of the screening symptoms of cough of any duration, fever of any duration, weight loss of any duration, or night sweats of ≥3 weeks in the preceding 4 weeks). TB laboratory investigations employed included chest radiograph, sputum AFB microscopy (three samples for each patient), complete blood count, erythrocyte sedimentation rate as well as pleural fluid analysis, abdominal ultrasound, or tissue histology where appropriate. To further improve reliability, before a diagnosis of TB was accepted, at least two physicians were required to agree on the diagnosis. All the laboratory investigations were carried out in the same center using the same protocol for specific investigations with standard quality assurance guidelines.
Definition of variables
Primary outcome: Tuberculosis diagnostic criteria Pulmonary tuberculosis
It was defined as the presence of cough with or without fever, weight loss, night sweats or hemoptysis, and demonstration of AFB in two or more sputum samples and/or chest radiological features compatible with TB.[21-23]
At the time of this study, the WHO definition of smear-positive TB as one or more AFB-positive smears had not been fully adopted at the study site; hence, the definition used in this study. We were unable to use either sputum mycobacterial culture or GeneXpert, which would have improved the sensitivity of TB diagnosis because these methods were not readily available at the time of this study which remains the case in most resource-limited settings. Moreover, in the Nigerian National TB management guideline, sputum mycobacterial culture is highly prioritized and is not yet indicated for routine diagnosis of TB irrespective of HIV status.[23]
Extra-pulmonary tuberculosis
It was defined as clinical features suggestive of TB in an extrapulmonary site without evidence of pulmonary involvement with any of the following: Compatible lymph node histology; exudative pleural effusion accompanied by a clinical response to anti-TB drugs; ultrasonography of the abdomen showing enlarged lymph nodes accompanied by a clinical response to anti-TB drugs.[21-23]
Disseminated tuberculosis
It was defined as clinical features suggestive of TB with concurrent involvement of at least two non contiguous organs, with positive sputum smear and/or histological and/or radiological evidence of TB.[21]
For patients with negative sputum AFB despite clinical and/or radiological features strongly suggestive of pulmonary TB as well as those having a clinical diagnosis of extrapulmonary TB, clinical response to only anti-TB drugs (resolution of symptoms/signs of TB, improvement of radiological abnormalities where applicable) within the 8 weeks of intensive phase of treatment was further required before diagnosis of TB was accepted.[13,24] In line with the protocol of the study site, all HIV-infected patients with lower respiratory infections and smear negative AFB are administered a 2 week course of broad spectrum antibiotics and only if there is no improvement are such patients considered for antituberculous therapy.
Highly active antiretroviral therapy adherence
It was assessed using both tablet counting and self-reporting methods. In the tablet counting method, pharmacy medication records for patients were matched by the pharmacist against the not-yet-used medicines brought to the pharmacy by the patients as a routine for refill of prescriptions, and the number of doses that ought to have been taken that were missed were recorded.[25] In the self-reporting method, patients were interviewed about their adherence over the previous day, previous week, and previous month successively.[26] If there was discrepancy between the two methods, the tablet counting method was used.
Adherence was defined as taking 95% of prescribed doses which corresponded to missing no >1 dose in a 10-day period (in 2 times a day dosing regimen), or one dose per month (in a once daily regimen).[25] Patients were therefore said to have HAART non-adherence if they missed >5% of their doses.[25,26] HAART adherence was reported as a dichotomous variable (i.e., yes or no).
Social class
It was assessed using the paternal occupation/income and the maternal educational attainment as recommended for Nigeria.[27] This method stratifies social class into five classes I to V. Class I represent the upper cadre, classes II and III the middle cadre, whereas classes IV and V are the lower cadre. Paternal occupation had a cumulative score of 3, whereas maternal educational attainment had a cumulative score of 2. The total score from both parameters placed each participant in the respective classes. This classification system is judged relevant in developing countries such as Nigeria where the mother’s education often influences healthcare knowledge and heath seeking behavior in the family irrespective of household income.
Ethical considerations
Ethical approval was obtained from the Ethics Committee of FMCO. Written informed consent was obtained from each patient before enrollment and confidentiality were ensured by every member of the research team. All patients newly diagnosed with TB were referred for appropriate treatment.
Data management and analysis
Data entry and analysis were carried out using the Epi Info version 3.5.1 statistical software (CDC, Atlanta, Georgia, 2008). Completed questionnaires were coded by numbers and double entered in the Epi Info software. Cross-checking and data cleaning were done. Data were presented as proportions for categorical variables. Quantitative variables were presented as mean (standard deviation) if they were normally distributed while non-normally distributed quantitative data were presented as median (interquartile range). The Student’s t-test was used to compare mean values of normally distributed quantitative variables, whereas non-normally distributed variables were compared using the Kruskal-Wallis test.
Bivariate comparisons utilized the Chi-square test as appropriate. Quantitative variables were dichotomized using cutoff points that had clinical relevance and that were as close as possible to the mean or median values in the entire population. For body mass index (BMI), the clinical relevance of 18.5kg/m2 as a cutoff point was considered more important than the mean value since TB usually presents with weight loss. A cutoff point of 200cells/µl was used for current CD4 count rather than the median value to better assess the impact of severe immunosuppression post-HAART.
Logistic regression model was used to determine the factors independently associated with prevalent TB using parameters that had a P < 0.1 on bivariate analysis. Statistical significance was set at P < 0.05.
Results
Characteristics of the study participants
so we included only 339 patients with complete data for the analysis [Figure 1]. In the final study population, 65.8% (222/339) were women. The mean age of the entire population was 41.1 (10.0) years. Urban dwellers constituted 56.3% (191/339) of the participants, and 50.4% (171/339) belonged to the lower social class. About one-fifth had a positive history of current alcohol use and 7.1% (24/339) ever smoked. Previous history of TB was documented in 23.6% (73/339) of patients. The most frequent WHO clinical stage at the time of HIV diagnosis was stage I comprising 41.0% (139/339) of patients followed by stage II comprising 31.3% (106/339) while the remaining 27.7% (94/339) presented at stages III or IV. About 49.6% (168/339) of the patients had baseline CD4 count <200 cells/µl, whereas the median current (post-HAART) CD4 count was 357 (211-496) cells/µl. The median durations of HIV diagnosis and HAART were 3.2 (2.0-5.0) years and 35.0 (20.0-50.0) months, respectively. HAART non-adherence was documented in 22.4% (76/339) of participants.
Table 1 shows the characteristics of the study participants according to TB status. There were statistically significant differences between the two groups in social class and HIV/ HAART/other clinical parameters with the exception of duration of HIV diagnosis.
| Characteristics | TB (n=26) | No TB (n=313) | P |
|---|---|---|---|
| Demographics | |||
| Male, n (%) | 13 (50.0) | 103 (32.9) | 0.08 |
| Age (years)† | 38.0 (6.6) | 41.4 (10.2) | 0.09 |
| Rural residence, n (%) | 13 (50.0) | 135 (43.1) | 0.50 |
| Lower social class | 19 (73.1) | 152 (48.6) | 0.02 |
| (4 and 5), n (%) | |||
| Health behavior, n (%) | |||
| Ever smoked | 4 (15.4) | 20 (6.4) | 0.09 |
| Current alcohol use | 6 (23.1) | 66 (21.1) | 0.08 |
| HIV severity and HAART | |||
| HIV duration (years)# | 2.7 (1.0-6.0) | 3.3 (2.0-5.0) | 0.61 |
| Baseline WHO clinical | 18 (69.2) | 76 (24.3) | <0.001 |
| stage III/IV, n (%) | |||
| HAART duration (months)# | 15.5 (6.0-36.0) 36.0 (22.0-52.0) | 0.02 | |
| HAART non-adherence, | 24 (92.3) | 52 (16.6) | <0.001 |
| n (%) | |||
| Baseline CD4 count | 90 (53-165) | 201 (119-267) | <0.001 |
| (cells/µl)# | |||
| Current CD4 count, | 165 (85-216) | 371 (233-523) | <0.001 |
| (cells/µl)# | |||
| Other clinical parameters | |||
| Previous TB, n (%) | 15 (57.7) | 58 (20.5) | <0.001 |
| BMI (kg/m2)† | 20.1 (4.0) | 24.8 (5.3) | <0.001 |
| Baseline haemoglobin† | 9.4 (2.2) | 11.4 (1.9) | <0.001 |
| Current haemoglobin† | 9.6 (2.2) | 11.8 (1.5) | <0.001 |
†Variables are expressed as means (SD), #Variables are expressed as median (IQR). BMI: Body mass index, TB: Tuberculosis, HAART: Highly active antiretroviral therapy, IQR: Interquartile range, SD: Standard deviation
Table 1: Characteristics of the study participants according to tuberculosis status
Prevalence and types of tuberculosis
In the entire study population, 12.7% (43/339) had a positive screening based on the TB screening algorithm used. Active TB was diagnosed in 7.7% (26/339) of patients. The 26 patients included 9 patients whose TB were diagnosed after initiation of HAART and were still on anti-TB drugs at the time of the study and 17 who were newly diagnosed during the study. Of these TB cases, 42.3% (11/26) had pulmonary TB, 34.6% (9/26) had disseminated TB, whereas the remaining patients had only extrapulmonary disease including tuberculous pleural effusion in 11.5% (3/26) of patients, TB lymphadenitis in 7.7% (2/26), and abdominal TB in 3.8% (1/26). In all, 20 patients had evidence of pulmonary involvement out of whom only 45% (9/20) had positive sputum smear.
Bivariate analysis for factors associated with prevalent tuberculosis
Bivariate analysis of factors associated with prevalent TB is presented in Table 2. All variables were dichotomized. Patients with TB were significantly more likely to belong to lower social class (P = 0.02). There was no statistically significant relationship between prevalent TB and age (P = 0.77), gender (P = 0.08), location of residence (P = 0.50), current alcohol use (P = 0.81), or having ever smoked (P = 0.09).
| Variable | OR (95% CI) | P |
|---|---|---|
| Demographic/health behavior factors | ||
| Age (=40 vs. >40 years) | 1.1(0.5-2.6) | 0.77 |
| Gender (male vs. female) | 2.0(0.9-4.6) | 0.08 |
| Social class (4 and 5 vs. 1-3) | 2.9(1.2-7.5) | 0.02 |
| Residence (Rural vs. Urban) | 0.7(0.3-1.7) | 0.50 |
| Ever smoked versus never smoked | 2.6(0.7-8.1) | 0.09 |
| Current alcohol use (yes vs. no) | 1.1(0.4-2.8) | 0.81 |
| HIV severity and treatment factors | ||
| Baseline WHO clinical stage (III/IV | 7.0(3.0-17.6) | <0.001 |
| vs. I/II) | ||
| HIV duration (<3 vs. =3 years) | 2.0(0.89-4.5) | 0.08 |
| HAART duration (<35 vs. =35 months) | 0.4(0.2-1.0) | 0.05 |
| HAART nonadherence (yes vs. no) | 59.2 (15.7-381.9) | <0.001 |
| Baseline CD4 count (<200 vs. | 29.5(5.4-620.0) | <0.001 |
| =200 cells/µl) | ||
| Current CD4 count (<200 vs. | 10.0(4.2-25.5) | <0.001 |
| =200 cells/µl) | ||
| Other clinical factors | ||
| BMI (<18.5 vs. =18.5 kg/m2) | 6.6(2.7-15.8) | <0.001 |
| Baseline hemoglobin (<10 vs.=10 g/dl) | 8.1(3.4-19.8) | <0.001 |
| Current hemoglobin (<10 vs. =10 g/dl) | 12.4(5.1-30.5) | <0.001 |
| Previous TB (yes vs. no) | 5.3(2.3-12.4) | <0.001 |
BMI: Body mass index, TB: Tuberculosis, HAART: Highly active antiretroviral therapy, OR: Odds ratio, WHO: World Health Organization, CI: Confidence interval
Table 2: Factors associated with prevalent tuberculosis in HIV-infected patients on highly active antiretroviral therapy (bivariate analysis)
A number of HIV severity and HAART-related parameters showed significant associations with prevalent TB including WHO clinical stage III/IV (P < 0.001) and HAART non-adherence (P < 0.001). Patients with TB were significantly more likely to have baseline CD4 count <200 cells/µl (P < 0.001) and currentCD4 count <200cells/µl (P < 0.001). Neither duration of HIV diagnosis <3 years (P = 0.08) nor HAART duration <35months (P = 0.05) had statistically significant association with prevalent TB.
Compared with patients without TB, those with TB had a higher proportion of individuals with past history of TB, BMI <18.5 kg/m2, baseline hemoglobin <10 g/dl, and current hemoglobin <10 g/dl (all P < 0.001).
Multivariate analysis for factors associated with prevalent tuberculosis
To identify factors independently associated with prevalent TB, we created a logistic regression model using factors that had P < 0.1 on bivariate analysis as shown in Table 3. The factors that were independently associated with prevalent TB were lower social class (P = 0.04), baseline CD4 count <200cells/µl (P = 0.02), HAART non-adherence (P < 0.001), previous TB (P < 0.01), and current hemoglobin <10 g/dl (P = 0.04).
| Variable | aOR (95% CI) | P |
|---|---|---|
| Gender (male vs. female) | 1.5(0.2-10.0) | 0.64 |
| Social class (4 and 5 vs. 1-3) | 31.7 (1.1-1417.3) | 0.04 |
| Ever smoked versus never smoked | 1.9(0.1-52.2) | 0.46 |
| Baseline WHO clinical stage | 0.2 (0.0-1.8) | 0.16 |
| (III/IV vs. I/II) | ||
| HIV duration (<3 vs. =3 years) | 0.1 (0.0-1.6) | 0.11 |
| HAART duration (<35 vs. =35 months) | 6.7(0.5-82.3) | 0.14 |
| HAART nonadherence (yes vs. no) | 125.5(9.6-1636.3) | <0.001 |
| Baseline CD4 count (<200 vs. | 31.0(1.6-590.6) | 0.02 |
| =200 cells/µl) | ||
| Current CD4 count (<200 vs. | 2.1(0.3-16.4) | 0.46 |
| =200 cells/µl) | ||
| BMI (<18.5 vs. =18.5 kg/m2) | 2.6(0.3-19.8) | 0.36 |
| Baseline hemoglobin (<10 vs. =10 g/dl) | 4.5(0.6-31.7) | 0.13 |
| Current hemoglobin (<10 vs. =10 g/dl) | 10.3 (1.1-99.2) | 0.04 |
| Previous TB (yes vs. no) | 13.8 (2.0-94.1) | <0.01 |
BMI: Body mass index, TB: Tuberculosis, HAART: Highly active antiretroviral therapy, OR: Odds ratio, aOR: Adjusted odds ratio, WHO: World Health Organization, CI: Confidence interval
Table 3: Factors associated with prevalent tuberculosis in HIV-infected patients on highly active antiretroviral therapy (multivariate analysis)
Discussion
We found a prevalence of active TB of 7.7% among HIV-infected patients who had received HAART for an average of 35 months in a high TB burden nation. Prevalent TB was associated with lower social class, severe immunosuppression before HAART initiation, HAART non-adherence, previous history of TB, and current hemoglobin <10 g/dl.
The findings of this study are considered important because identifying the high-risk groups for active TB as shown in this study may lead to a higher index of suspicion, closer follow-up, more intensive screening for TB, and earlier TB diagnosis with possibly improved outcome. In addition, addressing the factors associated with TB such as HAART non-adherence and lower social class may prove to be key strategies for improved prevention and control of active TB in patients receiving HAART at least in the context of developing countries with high TB burden. The issue of HAART adherence as a means of TB control in PLHIV deserves to be emphasized bearing in mind that HAART has generally improved the quality of life of PLHIV, and undeniably changed the epidemiology of TB for the better in countries with high prevalence of this co-infection.
The prevalence of TB documented in HIV patients on HAART in this study is comparable with findings in Brazil (6.8%-all forms of TB),[28] Tanzania (8.5%-pulmonary TB),[29] and Ethiopia (10.1%-all forms of TB).[30] The study in Brazil was a retrospective analysis of 599 HIV-positive patients out of whom 59% were on HAART. TB diagnosis was based on sputum smear positivity or mycobacterial culture or physician-diagnosed cases. The majority (73%) had pulmonary TB.[28] The study in Tanzania was cross-sectional and involved 233 patients of whom 74% were women. Children were included in the study though the mean age was 37 (10) years. About 15% had a past history of TB. TB was defined as cases positive for AFB by smear microscopy and/or culture.[29] In Ethiopia, a cross-sectional study of 385 HIV-positive patients was conducted with 91% on HAART, 64% women and mean age of 35.9 (9.2) years. About 27% of their patients had CD4 count <200cell/µl. The case definition of TB was not clearly stated.[30]
Some other high TB burden settings have reported lower rates of 3-5% (all forms of TB) in ART-experienced populations.[11,31] Although the case definitions of TB in those studies were comparable with that of our study, a possible reason for the difference in the reported rates of TB is that those studies involved much larger samples of 28,323 and 2514 subjects in the works of Nicholas et al. And Peck et al., respectively.[11,31]
On the other hand, some other studies have reported much higher rates of prevalent TB in patients receiving HAART: 17-27% in other settings in India that investigated only pulmonary TB[32,33] and 19.8% in Benin City, Nigeria.[34] There are some explanations for the disparity between these studies and ours. In the work of Giri et al.,[32] half of their participants were men, and over 70% had CD4 count <250cells/µl. The reason for the higher rate of prevalent TB in Benin City, Nigeria may be because only 70% of their participants were on HAART and understandably the remaining 30% who were HAART naïve had higher TB risk.
Generally, the availability of mycobacterial culture is another reason for variation in rates of diagnosed TB. For example, the prevalence documented for pulmonary TB by Ngowi.[29] in a setting in Tanzania where mycobacterial culture was employed in diagnosis in some patients was 3 times higher than the prevalence of all forms of TB documented by Peck et al.[31] in another Tanzanian setting where mycobacterial culture was not available.
The WHO estimated that only 3% of HIV-infected individuals developed TB in 2010 in Nigeria[14] although this data did not specify antiretroviral treatment status. Hospital-based studies have shown that the prevalence of active TB in predominantly HAART naive HIV-infected patients in Nigeria was 30-40% before antiretroviral drugs became readily available[35,36] and 6-14% in the post-HAART era.[37-39] Our finding on the prevalence of active TB among patients on HAART is therefore within the range described in the post-HAART era in other studies in Nigeria.
While there is evidence that TB can occur in HIV-infected patients at any level of CD4 count,[9] low CD4 count before HAART initiation has consistently been reported to be a risk factor for TB in patients on HAART.[7,8,10,37,38] According to Girardi et al.,[7] baseline CD4 count was associated with the occurrence of TB in Europe and North America from 6 months after initiation of HAART even after controlling for the 6-month CD4 count which was also predictive of TB. In South Africa, the risk of TB was independently associated with CD4 count <100cells/µl. In an evaluation of the relationship between TB and CD4 count in HIV-infected patients in another study in Nigeria, patients with CD4 count ≤200cells/µl were significantly more likely to have TB compared to those with values >200cells/µl.[37]
Similar to our finding, past history of TB has been reported to be a risk factor for active TB among patients on HAART in various studies in sub-Saharan Africa.[9,11,13,40] In a prospective cohort study in Coted’Ivoire, the risk of TB after HAART initiation was 2 times higher in patients with a past history of TB compared to those who had no past history of TB.[40] According to Komati et al.,[13] a history of TB at baseline was associated with subsequent TB and death during HAART in South Africa. Contrary to these reports, Lawn et al.[8] found that previous TB was not associated with subsequent TB in patients on HAART in another South African cohort. In that study, only 14% of the population had a past history of TB compared to about 24% in our study, so it is possible this contributed to the disparity.
While we found that low social class was independently associated with TB, Lawn et al.[8] reported that socioeconomic status was not a risk factor for TB in their population despite the fact that the proportion of individuals with low social class was similar in the two studies (51% in South Africa and 50.4% in our study). In line with our findings, HAART adherence <95% was identified as a strong predictor of TB in Mozambique.[41] Anemia has also been associated with TB in a number of studies.[10,11,13,30]
We observed that the significant association between either post-HAART CD4 count or WHO clinical stage III/IV and active TB seen on bivariate analysis was lost after controlling for confounders. Advanced WHO clinical stage at the time of HIV diagnosis[8,13,38,42-44] and low CD4 count post-HAART[7,10,12,32] have been shown in several studies to predict active TB in patients receiving HAART. Age, gender, and low BMI were not predictors of active TB in our study. The observation by Lawn et al.[8] that gender had no significant association with TB in South Africa despite a male predominance in their population agrees with our finding. Some other studies have reported younger age,[8,34] male gender,[11,20,39,42] and low BMI[10,11,13,29] to be significantly associated with TB disease in patients on HAART. It is possible that our study was underpowered to detect some of these associations. Another reason for the lack of association between WHO clinical stage and TB in our study may be the relatively low proportion of patients with advanced WHO clinical stage (27.7%).
It is also important to note that high HIV viral load both at baseline or during HAART has been strongly associated with active TB both in high and low TB burden countries.[7,10,13] We were unable to assess for the association between HIV viral load and prevalent TB due to lack of facilities for viral load quantification in our center at the time of this study which unfortunately is the case in most resource-limited settings.
Our study had a number of strengths. Selection bias was reasonably minimized by the use of a simple random sampling technique. The inclusion of only patients who had received HAART long enough to attain some immunological recovery and virological suppression mean that we did not recruit a group of HIV-infected patients who had unduly high risk of TB or unmasking TB immune reconstitution inflammatory syndrome. The TB diagnostic criteria, we adopted also minimized the chances of TB overdiagnosis.
Nevertheless, our observation should be interpreted in the light of our study limitations. Considering that the sample size was estimated based on a larger study on OIs, our study could have been underpowered to detect associations between prevalent TB and some other variables such as WHO clinical stage and post-HAART CD4count. We did not have facilities for sputum mycobacterial culture or GeneXpert at the time of this study either of which would have further improved the sensitivity of our TB diagnosis considering that our patients were HIV-infected and may present with atypical clinical features, unremarkable chest radiograph, and smear negative sputum despite having TB. Therefore, the actual prevalence of active TB in our HAART population may be higher than we found. Availability of mycobacterial culture for TB diagnosis remains a challenge in resource-limited settings. In fact, mycobacterial culture is highly prioritized for TB diagnosis in Nigeria and is not yet indicated for routine diagnosis irrespective of HIV status even in the 2014 revised National guideline.[23] The fact that we did not use more objective criteria such as Cage criteria for alcohol use and number of pack-years for cigarette smoking are also considered limitations in this study. This is worth mentioning considering that both chronic alcohol use and cigarette smoking are recognized risk factors for pulmonary infections.
Conclusion
This study has shown that prevalent TB remains an important public health problem among HIV-infected patients receiving HAART in high TB burden nations. Lower social class, previous TB, HAART non-adherence, severe immunosuppression before HAART initiation, and anemia post-HAART were factors independently associated with prevalent TB. We recommend that TB and HIV policy makers as well as clinicians should consider these findings to review TB management guidelines to raise the index of suspicion for TB diagnosis in these high-risk groups. Addressing key issues such as HAART non-adherence and poor living conditions in PLHIV in resource-limited settings is highly recommended as part of TB prevention strategy. The observation that baseline CD4 count below 200cells/µl was associated with prevalent TB underscores the importance of commencement of ART at higher CD4 count in line with current WHO recommendation.[45] Finally, the likelihood that the prevalence of active TB in our population may actually be higher than we found justifies a call for policy makers and relevant authorities in resource-limited settings such as Nigeria to make mycobacterial culture and molecular-based techniques such as GeneXpert readily available for TB diagnosis, especially in PLHIV.
Acknowledgment
This study was part of the thesis submitted to the School of Public Health, University of Western Cape, South Africa for the award of Master of Public health to MOI. Support for laboratory investigations was provided by the heart-to-heart clinic of FMCO.
We thank our patients for participating in this study. The clinical and administrative staff of the heart-to-heart clinic of FMCO is deeply appreciated for their assistance during data collection. We also wish to thank the resident doctors and house officers that assisted with patient recruitment. Cecy Patino-Suton of the American Thoracic Society Methods in Epidemiologic, Clinical, and Operations Research (ATS-MECOR) Global course is deeply appreciated for her input.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Ogoina D, Obiako RO, Muktar HM, Adeiza M, Babadoko A, Hassan A, et al. Morbidity and mortality patterns of hospitalised adult HIV/AIDS patients in the era of highly active antiretroviral therapy: A 4-year retrospective review from Zaria, Northern Nigeria. AIDS Res Treat 2012;2012:940580.
- Corbett EL, Marston B, Churchyard GJ, De Cock KM. Tuberculosis in sub-Saharan Africa: Opportunities, challenges, and change in the era of antiretroviral treatment. Lancet 2006;367:926-37.
- WHO. Global Tuberculosis Report (2013). Geneva, Switzerland: WHO; 2013.
- Santoro-Lopes G, de Pinho AM, Harrison LH, Schechter M. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis 2002;34:543-6.
- Babiker A, Darbyshire J, Pezzotti P, Porter K, Rezza G, Walker SA, et al. Changes over calendar time in the risk of specific first AIDS-defining events following HIV seroconversion, adjusting for competing risks. Int J Epidemiol 2002;31:951-8.
- Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: A cohort study. Lancet 2002;359:2059-64.
- Girardi E, Sabin CA, d’Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 2005;41:1772-82.
- Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: Long term incidence and risk factors in a South African cohort. AIDS 2005;19:2109-16.
- Bwana V, Tenu F, Magesa SM, Mfinanga SG. Smear positive pulmonary tuberculosis among HIV patients receiving highly active antiretroviral therapy in Dar es Salaam, Tanzania. Tanzan J Health Res 2011;13:14-20.
- Van Rie A, Westreich D, Sanne I. Tuberculosis in patients receiving antiretroviral treatment: Incidence, risk factors, and prevention strategies. J Acquir Immune Defic Syndr 2011;56:349-55.
- Nicholas S, Sabapathy K, Ferreyra C, Varaine F, Pujades-Rodríguez M; AIDS Working Group of Médecins Sans Frontières. Incidence of tuberculosis in HIV-infected patients before and after starting combined antiretroviral therapy in 8 sub-Saharan African HIV programs. J Acquir Immune Defic Syndr 2011;57:311-8.
- Akanbi MO, Achenbach CJ, Feinglass J, Taiwo B, Onu A, Pho MT, et al. Tuberculosis after one year of combination antiretroviral therapy in Nigeria: A retrospective cohort study. AIDS Res Hum Retroviruses 2013;29:931-7.
- Komati S, Shaw PA, Stubbs N, Mathibedi MJ, Malan L, Sangweni P, et al. Tuberculosis risk factors and mortality for HIV-infected persons receiving antiretroviral therapy in South Africa. AIDS 2010;24:1849-55.
- Federal Ministry of Health. National HIV/Syphilis Seroprevalence Sentinel Survey Among Pregnant Women Attending Antenatal Clinics in Nigeria. Department of Public Health National AIDS/STI Control Programme, 2010. Abuja, Nigeria; 2010.
- United States Embassy in Nigeria. Nigeria Tuberculosis Fact Sheet, 2012. Abuja, Nigeria; 2012. Available from: http:// www.photos.state.gov/libraries/nigeria/487468/pdfs/ JanuaryTuberculosisFactSheet.pdf. [Last accessed on 2016 Apr 02].
- Getahun H, Granich R, Sculier D, Gunneberg C, Blanc L, Nunn P, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: Barriers and solutions. AIDS 2010;24 Suppl 5:S57-65.
- National Population Comission of Nigeria. Population Census of the Federal Republic of Nigeria, 2006. Abuja, Nigeria: National Population Comission of Nigeria; 2006.
- Iroezindu MO, Ofondu EO, Hausler H, van Wyk B. Prevalence and risk factors for opportunistic infections in HIV patients receiving antiretroviral therapy in a resource-limited setting in Nigeria. J AIDS Clin Res 2013;S3:002. doi: 10.4172/2155-6113. S3-002.
- Mzileni MO, Longo-Mbenza B, Chephe TJ. Mortality and causes of death in HIV-positive patients receiving antiretroviral therapy at Tshepang Clinic in Doctor George Mukhari Hospital. Pol Arch Med Wewn 2008;118:548-54.
- Cain KP, McCarthy KD, Heilig CM, Monkongdee P, Tasaneeyapan T, Kanara N, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med 2010;362:707-16.
- Sharma SK, Kadhiravan T, Banga A, Goyal T, Bhatia I, Saha PK. Spectrum of clinical disease in a series of 135 hospitalised HIV-infected patients from North India. BMC Infect Dis 2004;4:52.
- Ghate M, Deshpande S, Tripathy S, Nene M, Gedam P, Godbole S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: Analysis by stages of immunosuppression represented by CD4 counts. Int J Infect Dis 2009;13:e1-8.
- Federal Ministry of Health. National tuberculosis, leprosy and buruli ulcer management and control guidelines. 6th ed. Abuja, Nigeria: Federal Ministry of Health; 2014.
- World Health Organization. Treatment of tuberculosis: Guidelines for national programs. Geneva, Switzerland: World Health Organization; 2003.
- Erah PO, Arute JE. Adherence of HIV/AIDS patients to antiretroviral therapy in a tertiary health facility in Benin city. Afr J Pharm Pharmacol 2008;2:145-52.
- Weiser S, Wolfe W, Bangsberg D, Thior I, Gilbert P, Makhema J, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr 2003;34:281-8.
- Olusanya O, Okpere EE, Ezimokhai M. The importance of socioeconomic class in voluntary fertility in developing countries. West Afr Med J 1985;4:205-7.
- Casseb J, Fonseca LA, Medeiros LA, Gonsalez CR, Lagonegro ER, Veiga AP, et al. Tuberculosis among HIV-1-infected subjects in a tertiary out-patient service in São Paulo city, Brazil. Rev Inst Med Trop Sao Paulo 2012;54:257-9.
- Ngowi BJ, Mfinanga SG, Bruun JN, Morkve O. Pulmonary tuberculosis among people living with HIV/AIDS attending care and treatment in rural Northern Tanzania. BMC Public Health 2008;8:341.
- Belay A, Alamrew Z, Berie Y, Tegegne B, Tiruneh G, Felek A. Magnitude and correlates of tuberculosis among HIV patients at Felege Hiwot Referral Hospital, Bahir Dar city, Northwest Ethiopia. Clin Med Res 2013;2:77-83.
- Peck RN, Luhanga A, Kalluvya S, Todd J, Lugoba S, Fitzgerald DW, et al. Predictors of tuberculosis in first 6 months after initiation of antiretroviral therapy: A case-control study. Int J Tuberc Lung Dis 2012;16:1047-51.
- Giri PA, Deshpande JD, Phalke DB. Prevalence of pulmonary tuberculosis among HIV positive patients attending antiretroviral therapy clinic. N Am J Med Sci 2013;5:367-70.
- Padyana M, Bhat RV, Dinesha M, Nawaz A. HIV-tuberculosis: A study of chest X-ray patterns in relation to CD4 count. N Am J Med Sci 2012;4:221-5.
- Okoh A, Omuemu V. Prevalence of HIV/AIDS and tuberculosis co-infection among patients in Benin city, Nigeria. Geneva Health Forum 2012. (Research project).
- Awoyemi OB, Ige OM, Onadeko BO. Prevalence of active pulmonary tuberculosis in human immunodeficiency virus seropositive adult patients in University College Hospital, Ibadan, Nigeria. Afr J Med Med Sci 2002;31:329-32.
- Salami AK, Katibi IA. Human immunodeficiency virus-associated tuberculosis: Pattern and trend in the University of Ilorin Teaching Hospital. Afr J Med Med Sci 2006;35:457-60.
- Nwabuko CO, Ejele OA, Chuku A, Nnoli MA, Chukwuonye II. Prevalence of tuberculosis-HIV coinfection and relationship between tuberculosis and CD4/ESR in HIV patients in Niger Delta Region of Nigeria. J Dent Med Sci 2012;2:1-4.
- Iliyasu Z, Babashani M. Prevalence and predictors of tuberculosis coinfection among HIV-seropositive patients attending the Aminu Kano Teaching Hospital, Northern Nigeria. J Epidemiol 2009;19:81-7.
- Olaniran O, Hassan-Olajokun RE, Oyovwevotu MA, Agunlejika RA. Prevalence of tuberculosis among HIV/AIDS patients in Obafemi Awolowo University Teaching Hospital Complex OAUTHC, Ile-Ife. Int J Biol Med Res 2011;2:874-7.
- Seyler C, Toure S, Messou E, Bonard D, Gabillard D, Anglaret X. Risk factors for active tuberculosis after antiretroviral treatment initiation in Abidjan. Am J Respir Crit Care Med 2005;172:123-7.
- Auld AF, Mbofana F, Shiraishi RW, Alfredo C, Sanchez M, Ellerbrock TV, et al. Incidence and determinants of tuberculosis among adults initiating antiretroviral therapy - Mozambique, 2004-2008. PLoS One 2013;8:e54665.
- Chu R, Mills EJ, Beyene J, Pullenayegum E, Bakanda C, Nachega JB, et al. Impact of tuberculosis on mortality among HIV-infected patients receiving antiretroviral therapy in Uganda: A prospective cohort analysis. AIDS Res Ther 2013;10:19.
- Wondimeneh Y, Muluye D, Belyhun Y. Prevalence of pulmonary tuberculosis and immunological profile of HIV co-infected patients in Northwest Ethiopia. BMC Res Notes 2012;5:331.
- Gudina EK, Gudissa FG. Prevalence of tuberculosis in HIV in Ethiopia in early HAART era: Retrospective analysis. Pan Afr Med J 2013;14:126.
- World Health Organization. Consolidated Guideline on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Recommendations for a Public Health Approach. Geneva, Switzerland: World Health Organization; 2013.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.