Factors Influencing Endometrial Thickness in Postmenopausal Women
- *Corresponding Author:
- Dr. Shripad Hebbar
Department of Obstetrics and Gynecology, Kasturba Medical College, Manipal University, Udupi District - 576 104, Karnataka, India.
E-mail: drshripadhebbar@yahoo.co.in
Abstract
Background: Cut‑off values for endometrial thickness (ET) in asymptomatic postmenopausal woman have been standardized. However, there are no comprehensive studies to document how various factors can influence the ET after the age of menopause. Aim: To study the various factors influencing the ET in postmenopausal women. Subjects and Methods: This was a prospective observational study. A total of 110 postmenopausal women underwent detailed history taking, clinical examination, and transvaginal scan for uterine volume and ovarian volume. The volumes were calculated by using ellipsoid formula: Width × thickness × height × 0.523. The variation in ET with respect to the influencing factors such as age, duration of menopause, parity, body mass index (BMI), medical illness like diabetes/hypertension, drugs like tamoxifen, presence of myoma, uterine volume, ovarian volume, and serum estradiol (in selected patients) were measured. Descriptive analysis was performed using SPSS software (version 16, Chicago II, USA) to obtain mean, standard deviation (SD), 95% confidence intervals (CIs) and inter quartile ranges. Comparison of means was carried out using analysis of variance. Results: The mean (SD) age of the patients was 55.4 (6.91) years (95% CI, 54.1, 56.7). The mean (SD) age at menopause was 47.95 (3.90) years (95% CI, 47.2, 48.7) and the mean (SD) duration of menopause was 7.27 (6.65) years (95% CI, 6.01, 8.53). The mean (SD) ET was 3.8 (2.3) mm (95% CI, 3.36, 4.23). Medical illness like diabetes and hypertension did not alter the ET. ET increased as BMI increased and it was statistically significant. The presence of myoma increased uterine volume significantly and was associated with thick endometrial stripe. Similarly, whenever the ovaries were visualized and as the ovarian volume increased, there was an increase in ET. When ET was > 4 mm (n = 37), they were offered endocel, of which 16 agreed to undergo the procedure. None were found to have endometrial cancer. Conclusion: This study suggests that parity, BMI, presence of myoma, tamoxifen usage, uterine volume, ovarian volume and serum estradiol influence the ET in postmenopausal women.
Keywords
Diabetes, Endometrial thickness, Hypertension, Ovarian and uterine volume, Postmenopause
Introduction
The menopause is the time when permanent cessation of menstruation occurs following the loss of ovarian activity. Clinically, menopause is defined retrospectively as the time of the final menstrual period followed by 12 months of amenorrhea. Postmenopause describes the period following the final menses.[1] It is well-known that the endometrium irrespective of reproductive or menopausal status contains estrogen receptors and responds to circulating estrogens. Abundance of estrogen results in endometrial overgrowth and vice versa, the low levels bring about endometrial atrophy. The extent of endometrial thickness (ET) constitutes a potential biomarker of estrogen status even in postmenopausal women.[2]
Though ovaries are the predominant source of estrogen, peripheral adipose tissue also contributes to its synthesis and is influenced by medical disorders like metabolic X syndrome.[3] Obese diabetics have high insulin resistance and as a result have high plasma insulin levels, which may increase free estrogen levels by decreasing the concentration of sex hormone-binding globulin.[4] Insulin growth factor (IGF-1) and its binding protein (IGF binding protein-1) are known to promote endometrial cell growth.[5] Elevated levels of IGF found in women with higher body mass index (BMI) may produce endometrial hyperplasia.
However, asymptomatic endometrial thickening found on ultrasound examination in postmenopausal women often poses a clinical management dilemma.[6,7] Although the prevalence of endometrial cancer is relatively low in women with no vaginal bleeding, the disease has the best outcome when it is detected at an early stage. The diagnosis is straight forward and be picked up in early stage, when postmenopausal women present with bleeding.
Endometrial thickness after menopause may indicate malignancy when it is more than >4-5 mm.[8,9] Nevertheless, there may be other influencing factors such as age,[2] menopausal years,[10] parity,[2] BMI, medical illness like diabetes[11]/hypertension,[12] drugs like tamoxifen[13]/hormone replacement therapy (HRT), myoma, uterine volume,[14] ovarian volume[14] and serum estradiol.[2] These factors exert their influence on the endometrium and the resultant changes to some extent may be picked up by sonographic evaluation. The following study aims at the determination of ET by transvaginal ultrasound (TVS) and its variation in relation to different influencing factors.
Subjects and Methods
This was a prospective observational study to determine the influence of various factors on ET for the duration of 1 year (January 2011-December 2011). The patients were included in this study who were either out-patients or in-patients presenting to the Department of Obstetrics and Gynecology, Kasturba Medical College, Manipal and also from other departments for example, medicine and medical oncology who were referred to us for gynecological evaluation. Institutional ethical committee clearance was obtained at the start of the study and patients were recruited after taking informed consent. Inclusion criteria were postmenopausal women with an intact uterus and no vaginal bleeding. Exclusion criteria were patients with ovarian tumor, cancer endometrium, and cervical cancer. All patients satisfying the above inclusion criteria were enrolled in the study and detailed history regarding parity, age at menopause, duration of menopause (years since menopause [YSM]), associated medical illnesses (diabetes mellitus, and hypertension), and drug intake (tamoxifen for breast cancer) was elicited. Height, weight and blood pressure were recorded. Then the patients were subjected to routine gynecological check-up where abdominal and pelvic examination was performed, followed by TVS for uterine volume, ovarian volume and ET. All the scans were done by either first author or the last author (first author has 15 years of experience in transvaginal scan and the last author who has 7 years of experience) and 5.0 MHz vaginal probe (equipment model: Philips HD11XE) was used for ultrasound measurements.
Sample size estimation
Warming et al.[15] conducted a cross-sectional study on 1182 asymptomatic healthy postmenopausal women and measured ET transvaginally. They reported that mean ET in them was 2.1 mm with a standard deviation (SD) of 1.4 mm. We estimated sample size required to show the significant difference in means at 0.5 mm, 1 mm and 1.5 mm, with a desired level of power of 90% and level of significance 0.05, by using the formula:
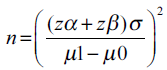
Where
zα = 1.96 (critical value that divides the central 95% of z distribution from 5% in the tails),
zβ = 1.28 (critical value that separates the lower 10% of distribution from upper 90%),
σ = SD, μ1-μ2 = difference of two means.
Accordingly, it was estimated that 82 patients are required to show the difference of 0.5 mm from established mean, 21 are required to show the difference of 1 mm and 10 patients required to show the difference of 1.5 mm.
We could achieve the sample size of 110 patients during the study period. We restricted serum estradiol estimation to a subset of 32 patients considering the cost of investigation and this number far exceeded minimum sample size required for statistical comparison.
Endometrial thickness was measured in the longitudinal plane and included the measurement between the two basal layers of the anterior and posterior uterine wall at the thickest point. Uterus was measured longitudinally and transversely measuring the uterocervical length, antero-posterior (AP) diameter and transverse diameter. Uterine volume was calculated by 0.523 × AP diameter × transverse diameter × utero-cervical length. Similarly ovarian volume was measured longitudinally, transversely and producing a sector of 90° with the probe the depth was measured. Ovarian volume was calculated by width × thickness × height × 0.523 of each ovary, right, and left respectively.[12]
The variables studied were age, menopausal years (YSM), parity, and presence of diabetes/hypertension, BMI, tamoxifen therapy, uterine volume, presence of fibroids, ovarian volume and serum estradiol. For BMI, quetlet index was used which was calculated as weight in kg/m2. They were divided into four groups based on BMI as ≤20, 20.1-25, 25.1-30, >30 (kg/m2) defined as underweight, normal, overweight, and obese respectively. Women were divided into two groups based upon serum estradiol levels (Group I ≤ 20 pg/ ml, Group II > 20 pg/ml.
Data were analyzed using SPSS software (version 16, Chicago II, USA). Descriptive statistics was used to calculate mean, SD, 95% confidence intervals (CIs) and inter quartile ranges (IQRs). Comparison of means was carried out using analysis of variance and P < 0.05 was considered to be significant.
Results
A total of 110 postmenopausal patients were studied. The basic characteristics (mean, SD, 95% CIs, range and IQR) of the postmenopausal women are shown in Table 1. The mean age at the time of presentation of was 55.4 years. The mean menopausal age was 47.95 years and duration of menopause (YSM) was 7.27 years. The presenting complaints were vague abdominal pain (15.5%, 17/110), mass descending per vaginum (15.5%, 17/110), back ache (13.6%, 15/110), dysuria (9.1%, 10/110), carcinoma of breast for gynecological evaluation (6.4%, 7/110), hot flashes (5.5%, 6/110), vaginal discharge (4.5%, 5/110), pruritus vulva (3.6%, 4/110) and dyspareunia (2.7%, 3/110). About 23.6% (26/110) of patients came for regular health check-up. It was observed that as the age increased from 41 years to 70 + years, there was a decrease in ET from 5.01 mm to 3.10 mm. There was also significant decrease in ET as the duration of menopause (YSM) increased. IQR was taken for uterine and ovarian volume as they had extremes of values.
| Parameter | Mean (SD) | 95% CI | Range | |
| Patient characteristics | ||||
| (n=110) | ||||
| Age (years) | 55.4 (6.91) | 54.1, | 56.7 | 40-76 |
| Age at menopause (years) | 47.95 (3.90) | 47.2, | 48.7 | 39-55 |
| YSM | 7.27 (6.65) | 6.01, | 8.53 | 1-25 |
| BMI (kg/m2) | 25.19 (4.04) | 24.41, | 25.93 | 16.2-37.5 |
| Parameter | Mean (SD) | Range | IQR | |
| Uterine (n=88) and | ||||
| ovarian (n=81) volume | ||||
| characteristics | ||||
| Uterine volume excluding | 26.36 (16.77) | 6.5-79.16 | 15, 35 | |
| patients with myoma (cm3) | ||||
| Ovarian volume (cm3) | 1.93 (2.41) | 0.08-14.74 | 0.08, 1.83 | |
YSM: Years since menopause, BMI: Body mass index, SD: Standard deviation, IQR: Inter quartile range, CI: Confidence interval
Table 1: Demographic data
Of these 110 women, 5.5% (6/110) were nulliparous, where as 94.5% (114/110) women had 1-4+ children. About 23% (25/110) of study subjects were diabetic. Though diabetes is a part of metabolic X syndrome, in this study, it was found that there was no significant difference in ET between the diabetics and nondiabetics. In this study, 39% (43/110) of the patients were hypertensive and surprisingly they had lower ET compared with normotensives, but the difference was not statistically significant (P = 0.78). The women were divided into four groups according to BMI. Of these 11.8% (13/110) were underweight, 34.5% (38/110) were normal, 42.7% (47/110) were overweight and 10.9% (12/110) were obese. It was observed that as the BMI increased there was an increase in ET. It was interesting to note that more than 50% of our subjects had BMI more than 25.
In the study group, there were seven patients (6.3%) who suffered from carcinoma of the breast, were on tamoxifen for 2 years. Their age ranged from 42 to 58 years (mean age 50.14 years). Though there was an increase in ET compared to normal people (5.24 vs. 3.97 mm), it was not statistically significant as numbers in tamoxifen was low.
We could not visualize ovaries in 26.4% (29/110) of patients. However, whenever the ovaries were visualized that is 49.1% (54/110) of cases both ovaries could be imaged and in 24.5% (27/110), only one ovary was seen), the ET was statistically significant. Similarly as the ovarian volume increased from <1 cm3 to more than 3 cm3 there was an increase in ET.
In 21 patients myomas (intramural-18, subserosal-3, submucus-0) were found incidentally and it was found that the presence of myoma positively correlated with ET. Patients without myoma (80.7%, [88/110] of total subjects) were divided further into three groups based on quartile values [that is <25th, 25th to 50th, >50th centiles, these centile values are given in Table 1] of uterine volumes as ≤15, 15.1-35, >35 cm3. It was observed that the increase in uterine volume was associated with an increase in ET showing a positive correlation. We also observed that irrespective of the presence or absence of myoma, the ET was more when the uterine volume was more. The variations in ET withrespect to various influencing factors are shown in Table 2.
| Factors | n (%) | Mean endometrial thickness in mm (mean (SD)) | 95% CI | P value |
|---|---|---|---|---|
| Age (years) | ||||
| 41-50 | 32 (29.1) | 5.01 (3.0) | 3.92, 6.10 | 0.02* |
| 51-60 | 59 (53.6) | 3.71 (1.6) | 3.29, 4.13 | |
| 61-70 | 17(15.5) | 3.21 (2.5) | 1.95, 4.47 | |
| >70 | 2(1.8) | 3.10 (1.7) | # | |
| Menopausal years (YSM) | ||||
| 1-5 | 68(61.8) | 4.70 (2.4) | 4.12, 5.28 | <0.01* |
| 6-10 | 19(17.3) | 3.06 (2.1) | 2.04, 4.08 | |
| >10 | 23(20.9) | 2.74 (1.4) | 2.15, 3.33 | |
| Parity | ||||
| No child | 06 (5.5) | 6.25 (2.1) | 4.10, 8.40 | 0.03* |
| 1 child | 12(10.9) | 4.34 (2.3) | 2.90, 5.78 | |
| 2-4 children | 74(67.3) | 4.00 (2.3) | 3.46, 4.54 | |
| More than 4 children | 18(16.4) | 3.10 (2.1) | 2.06, 4.14 | |
| BMI (kg/m2) | ||||
| <20 | 13(11.8) | 3.04 (1.4) | 2.17, 3.91 | <0.01* |
| 20.1-25 | 38(34.5) | 3.80 (2.4) | 3.02, 4.58 | |
| 25.1-30 | 47(42.7) | 3.89 (1.9) | 3.34, 4.44 | |
| >30 | 12(10.9) | 6.20 (3.4) | 4.03, 8.37 | |
| Medical illness | ||||
| Diabetics | 25 (23) | 4.48 (2.5) | 3.45, 5.51 | 0.30 |
| Nondiabetics | 85 (77) | 3.92 (2.3) | 3.43, 4.41 | |
| Hypertensives | 43 (39) | 3.90 (2.6) | 3.10, 4.70 | 0.78 |
| Normotensives | 67 (61) | 4.10 (2.2) | 3.57, 4.63 | |
| Tamoxifen therapy | ||||
| Carcinoma breast patients on tamoxifen | 7(6.3) | 5.20 (2.0) | 3.37, 7.03 | 0.16 |
| Normal postmenopausal women | 103 (93.7) | 3.90 (2.3) | 3.44, 4.36 | |
| Uterine volume (cm3) | ||||
| ≤15 | 20(22.7) | 2.90 (1.4) | 2.24, 3.56 | <0.01* |
| 15.1-35 | 47(53.4) | 3.08 (1.4) | 2.68, 3.48 | |
| >35 | 21(23.9) | 5.33 (2.8) | 4.06, 6.60 | |
| Presence of myoma | ||||
| Myoma | 22(19.3) | 5.90 (3.1) | 4.51, 7.29 | <0.01* |
| No myoma | 88(80.7) | 3.54 (1.8) | 3.16, 3.92 | |
| Ovarian status | ||||
| Both ovaries seen | 54(49.1) | 4.60 (2.6) | 3.89, 5.31 | <0.01* |
| One ovary seen | 27(24.5) | 3.90 (2.1) | 3.07, 4.73 | |
| Ovaries not seen | 29(26.4) | 3.05 (1.5) | 2.46, 3.64 | |
| Ovarian volume (cm3) | ||||
| >3 | 16(14.5) | 5.70 (2.4) | 4.43, 6.97 | <0.01* |
| 1-3 | 28(25.5) | 4.20 (2.6) | 3.21, 5.19 | |
| <1 | 37(33.6) | 3.80 (2.4) | 3.02, 4.58 | |
| Not visualized | 29(26.4) | 3.05 (1.5) | 2.46, 3.64 | |
| Serum estradiol (picograms/ml) | ||||
| ≤20 | 23(71.8) | 3.60 (2.4) | 2.57, 4.63 | 0.13 |
| >20 | 09(28.2) | 5.04 (2.3) | 3.26, 6.82 |
*Significant, #No. too small for CI estimation. CI: Confidence interval, YSM: Years since menopause, BMI: Body mass index, SD: Standard deviation
Table 2: Endometrial thickness according to various influencing factors (total number of patients=110)
Endometrial thickness was compared in two groups of women based upon serum estradiol (mentioned in materials and methods). Thickness was found to be high when the serum estradiol levels were high. The BMI was also found to be high with high serum estradiol levels (mean [SD], 24.5 [5.04] kg/m2) compared with low serum estradiol group (mean [SD], 23.4 [3.67] kg/m2), however this difference did not reach statistical significance (P = 0.52).
In this study, the cut-off for ET of postmenopausal women was taken as 4 mm for the purpose of diagnostic evaluation.[16,17] All postmenopausal women who had ET more than 4 mm (n = 37) were offered endocel. Of these 37 patients, only 16 accepted and had endometrial sampling. Twenty one patients were not willing for endocel, but were willing for regular followup. The various pathology reports and followup patients are shown in Table 3.
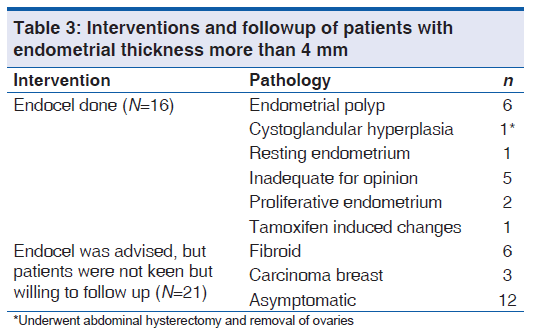
Of the 16 patients who had endocel, six patients had endometrial polyp, one had cystoglandular hyperplasia who underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy as she had other associated risk factors such as diabetes and hypertension. In five patients, specimen was inadequate for opinion. In one patient, who was a case of carcinoma of breast on tamoxifen, the histopathology was reported as tamoxifen induced changes.
Discussion
In this study, 110 postmenopausal women were enrolled and their ET was measured. The various influencing factors of ET such as age, menopausal years, parity, BMI, medical illness like diabetes/hypertension, drugs like tamoxifen, fibroids, uterine volume, ovarian volume, and serum estradiol were studied. We found that the mean ET decreased as YSM increased. This may be probably due to fall in hormone levels, mainly estrogen as age and menopausal years increase. The decreasing trends in ET with progressive increase in duration of menopause was also noted by Warming et al.[15] They found that during the first 5 years after menopause (YSM) the mean ET was 2.3 mm, but it decreased by 0.03 mm/year (P < 0.01). From 5 to 13 YSM the ET remained stable at a mean of 1.8 mm with no significant changes (P = 0.13). However, the mean ET in our study in women with duration of menopause <5 years was 4.7 mm which is higher compared with their observation. This finding probably is due to the fact that, the mean age of patients belonging to this group in our study was lesser compared with their study (mean [SD] 51.2 (4.2) vs. 54.1 (3.0) and 27.9% (19/68) patients belonging to this group has associated ultrasound finding of myoma. Tsuda et al. reported that YSM was a significant variable associated with ET.[18] However, Gull et al. in his study reported that mean ET between women with ≤5 YSM and >5 YSM did not differ significantly (mean [SD] 3.5 [0.2] vs. 3.4 [0.1], P > 0.05).[19]
We observed that in nulliparae, the ET was more (6.25 mm), which may be due to long periods of exposure to unopposed estrogen with no disruption of normal menstrual cycle.[19] Gull et al. in their study, they found that the number of completed pregnancies was associated to have an inverse correlation to endometrial disease and an increasing number of pregnancies was thought to provide protection against endometrial hyperplasia and cancer.[19] Albrektsen et al. and Lambe et al.,[20,21] suggested that mechanical shedding of precursor cells of malignant potential at each delivery prevents thickened endometrium and related disorders in subsequent years. Another explanation given is that antimitotic property of progesterone is supposed to have growth limiting effect over endometrium.
Usually, it is said that diabetes and hypertension increase the ET in postmenopausal women as they are part of metabolic X syndrome. In their study Alcázar et al. found that there was a significant change in ET in in hypertensive women.[22] Bornstein et al. further investigated hypertensive women receiving medications and those who were not on treatment.[11] They found that ET was more with the use of antihypertensive drugs, but not so when they were on no medications. This phenomenon was attributed to be due to trophic effect of antihypertensive drug, beta blockers in particular, on uterine endometrium.[23] In this study, it was found that the ET was not influenced by hypertension. This finding was comparable to earlier studies made by Serdar Serin et al. and Gürbüz et al.[24,25].
It would have been ideal, if we too had investigated in depth about the effect of different antihypertensive drugs, duration of therapy on uterine ET.
Hypertension and diabetes have known to be risk factors for endometrial cancer too. They are part of corpus cancer syndrome. Hence, it is logical to think that they may affect the ET positively. In addition, most of these women tend to be obese, which again may bring about increase in ET. However, Gull et al. in their study, they found that medical illness like diabetes; hypertension did not influence the ET.[19]
In this study increase in BMI was associated with an increase in ET. This may be to increased peripheral conversion of androstenedione by aromatization in obese postmenopausal women. Van den Bosch et al. made a cross-sectional study consisting of 167 consecutive postmenopausal women referred for vaginal ultrasonographic evaluation.[26] Age, weight, and BMI showed a significant positive correlation with ET. Nakamura et al. found only 15 of 242 in his series had BMI >25 and concluded that BMI is not a risk factor for endometrial thickening in Japanese women (ET in obese and nonobese women 2.2 mm vs. 1.5 mm, P = 0.27).[27]
Tamoxifen belongs to selective estrogen receptor modulators group of drugs. It has opposite actions on breast (suppressive) and uterus (proliferative). Its antiestrogenic property has therapeutic effects in breast cancer, however the dosage used in the treatment of breast cancer brings about modest estrogenic activity on uterine endometrium resulting in endometrial proliferation, hyperplasia, polyp formation and even endometrial carcinoma and sarcoma.[17] Cheng et al. found a significant increase in ET and abnormal endometrial histopathologic findings in postmenopausal tamoxifen treated women.[28] Fishman et al. found that ET increased with increasing duration of tamoxifen use at a rate of 0.75 mm/year.[29]
According to this study, ET was increased in women with fibroids compared with women without fibroids. This is in comparison with the study by Gull et al.[19] They hypothesized that the concentrations of estrogen and progestogen receptors are relatively high in women with fibroids compared to those without fibroids. Hence, women with uterine fibroids exhibit exaggerated response to circulating estrogen, which results in increased ET. It is now understood that estrogen and progesterone receptors as well as other growth factors play an important role in regulating endometrial and myometrial growth. Our study too showed that the endometrium is likely to be thicker in the presence of myoma and when bilateral ovaries were visualized by TVS, thereby indicating the probability of hyperoestrogenic state.
Sit et al. studied the relation between ET and various influencing factors in 1271 women ages 55-74 years who underwent TVS screening as part of the prostate, lung, colorectal and ovarian cancer screening trial.[2] Factors associated with increased ET included lower age, unmarried status, history of uterine fibroid, current HRT use, fewer YSM, history of hypertension, and increased BMI.
Sherman et al. concluded that large ovaries represent a marker of higher androgen levels, indicating greater availability of substrate for estrogen synthesis in peripheral adipose tissue, which could predispose to endometrial hyperplasia.[30] They may represent a marker of risk for hormonally related tumors. It was also found that when ovarian volume was more than 3 cm3, the endometrial disease was more. Though, there were no studies comparing uterine volume and ET, in this study, there was increase in ET as the uterine volume increased.
It was observed that the ET was more when the serum estradiol was more. This was because their BMI was more, so there was peripheral conversion of androstenedione to oestrogen in adipocytes. It was also observed that as age increased there was decrease in ovarian volume resulting in estrogen paucity and endometrial atrophy.
We found 37 (33.6%) cases of increased ET (>4 mm), but none had endometrial cancer. When endocel was done in 16 patients, 37.5% of them had a polyp and in 31.2% the sample was inadequate. Soha Siam et al. evaluated 261 asymptomatic postmenopausal women with ET ≥5 mm for evidence of abnormal endometrial pathology.[8] They found that validity tests to predict abnormal endometrium at different cut off values for ET showed that cut-off values of 5 and 6 mm had the highest sensitivity while that of 11 and 12 mm had the highest specificity. They concluded that although ET was significantly higher in asymptomatic postmenopausal women with abnormal endometrial histopathology, there is no single cut-off value that has accepted high sensitivity and specificity and the need for further evaluation for these cases is not routinely advised.
Conclusion
This study suggests that ET in postmenopausal women may be influenced by various factors such as parity, BMI, drugs like tamoxifen, presence of myoma, uterine volume, ovarian volume and serum estradiol. Hence these factors have to be taken into consideration, while evaluating these women.
Strengths and limitations of the study
• Most of the available studies focus on endometrial changes in postmenopausal women, whereas in this study thorough ultrasound assessment of genital organs has been done by calculating uterine and ovarian volume, in addition to determination of ET and correlation has been established
• Though in general, the total number of patients required to conduct the study far exceeded the required minimum sample size, there were less number of patients in some of the subcategories, for example; cancer breast patients receiving tamoxifen therapy
• When ET was more than 4 mm (n = 37), only 16 accepted and had endometrial sampling. If all of them had agreed for the procedure, it would have sizably improved the strength of histopathological findings, so as to frame a guideline for management of asymptomatic postmenopausal endometrial thickening.
Acknowledgement
This study was possible because of institutional research grant provided by Manipal University. The authors would like to thank Dr. Pratap Kumar, Unit 1 Chief, Dr. Muralidhar Pai, Unit 2 chief, Dr. Jyothi Shetty Unit 4 chief and Dr. Sapna Amin, Unit 5 chief for permitting to conduct study on some of their patients.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril 2001;76:874-8.
- Sit AS, Modugno F, Hill LM, Martin J, Weissfeld JL. Transvaginal ultrasound measurement of endometrial thickness as a biomarker for estrogen exposure. Cancer Epidemiol Biomarkers Prev 2004;13:1459-65.
- Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab 1991;72:83-9.
- Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes Control 2000;11:185-92.
- Augustin LS, Dal Maso L, Franceschi S, Talamini R, Kendall CW, Jenkins DJ, et al. Association between components of the insulin-like growth factor system and endometrial cancer risk. Oncology 2004;67:54-9.
- Gambacciani M, Monteleone P, Ciaponi M, Sacco A, Genazzani AR. Clinical usefulness of endometrial screening by ultrasound in asymptomatic postmenopausal women. Maturitas 2004;48:421-4.
- Wolfman W, Leyland N, Heywood M, Singh SS, Rittenberg DA, Soucy R, et al. Asymptomatic endometrial thickening. J Obstet Gynaecol Can 2010;32:990-9.
- Soha Siam, Azza A. Abd El-Hameed. Thickened endometrium in asymptomatic postmenopausal women: Is biopsy mandatory? Med J Cairo Univ 2011;79:723-7.
- Goldstein SR. Modern evaluation of the endometrium. Obstet Gynecol 2010;116:168-76.
- Andolf E, Dahlander K, Aspenberg P. Ultrasonic thickness of the endometrium correlated to body weight in asymptomatic postmenopausal women. Obstet Gynecol 1993; 82:936-40.
- Bornstein J, Auslender R, Goldstein S, Kohan R, Stolar Z, Abramovici H. Increased endometrial thickness in women with hypertension. Am J Obstet Gynecol 2000;183:583-7.
- Garuti G, Cellani F, Centinaio G, Sita G, Nalli G, Luerti M. Baseline endometrial assessment before tamoxifen for breast cancer in asymptomatic menopausal women. Gynecol Oncol 2005;98:63-7.
- Adeeb N, Nur-Azurah AG, Ong FB, Seri SS, Shamsuddin K, Noor-Aini MY. Normograms of ovarian volume, uterine size and endometrial thickness in urban midlife Malaysia women. Med Health 2007;2:66-79.
- Warming L, Ravn P, Skouby S, Christiansen C. Measurement precision and normal range of endometrial thickness in a postmenopausal population by transvaginal ultrasound. Ultrasound Obstet Gynecol 2002;20:492-5.
- Gull B, Karlsson B, Milsom I, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol 2003;188:401-8.
- American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 426: The role of transvaginal ultrasonography in the evaluation of postmenopausal bleeding. Obstet Gynecol 2009;113:462-4.
- Tsuda H, Kawabata M, Kawabata K, Yamamoto K, Umesaki N. Improvement of diagnostic accuracy of transvaginal ultrasound for identification of endometrial malignancies by using cutoff level of endometrial thickness based on length of time since menopause. Gynecol Oncol 1997;64:35-7.
- Gull B, Karlsson B, Milsom I, Granberg S. Factors associated with endometrial thickness and uterine size in a random sample of postmenopausal women. Am J Obstet Gynecol 2001;185:386-91.
- Albrektsen G, Heuch I, Tretli S, Kvåle G. Is the risk of cancer of the corpus uteri reduced by a recent pregnancy? A prospective study of 765,756 Norwegian women. Int J Cancer 1995;61:485-90.
- Lambe M, Wuu J, Weiderpass E, Hsieh CC. Childbearing at older age and endometrial cancer risk (Sweden). Cancer Causes Control 1999;10:43-9.
- Alcázar JL. Endometrial sonographic findings in asymptomatic, hypertensive postmenopausal women. J Clin Ultrasound 2000;28:175-8.
- Okman-Kilic T, Kucuk M. The effects of antihypertensive agents on endometrial thickness in asymptomatic, hypertensive, postmenopausal women. Menopause 2003;10:362-5.
- Serdar Serin I, Ozçelik B, Basbug M, Ozsahin O, Yilmazsoy A, Erez R. Effects of hypertension and obesity on endometrial thickness. Eur J Obstet Gynecol Reprod Biol 2003;109:72-5.
- Gürbüz B, Yalti S, Yildirim G. Endometrial thickness and uterine size in postmenopausal women. Int J Gynaecol Obstet 2004;84:268-70.
- Van den Bosch T, Vandendael A, Van Schoubroeck D, Lombard CJ, Wranz PA. Age, weight, body mass index and endometrial thickness in postmenopausal women. Acta Obstet Gynecol Scand 1996;75:181-2.
- Nakamura H, Tsuda H, Hosoi M, Sato T, Inoue T, Nishimura S, et al. Endometrial thickness in Japanese women withhypertension or/and type 2 diabetes mellitus. Eur J Obstet Gynecol Reprod Biol 2006;129:174-7.
- Cheng WF, Lin HH, Torng PL, Huang SC. Comparison of endometrial changes among symptomatic tamoxifen-treated and nontreated premenopausal and postmenopausal breast cancer patients. Gynecol Oncol 1997;66:233-7.
- Fishman M, Boda M, Sheiner E, Rotmensch J, Abramowicz J. Changes in the sonographic appearance of the uterus after discontinuation of tamoxifen therapy. J Ultrasound Med 2006;25:469-73.
- Sherman ME, Lacey JV, Buys SS, Reding DJ, Berg CD, Williams C, et al. Ovarian volume: Determinants and associations with cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev 2006;15:1550-4.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.