Feasibility of Hypofractionated Radiotherapy for Locally Advanced Cancer Esophagus
2 Department of Radiation Therapy and Oncology, Government Medical College and Hospital, Nagpur, India
Citation: Rohit Santosh Kabre. Feasibility of Hypofractionated Radiotherapy for Locally Advanced Cancer Esophagus. Ann Med Health Sci Res. 2017; 7: 23-28
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Context: Concurrent chemo radiotherapy is standard of care for locally advanced cancer esophagus despite showing 50% local failures. Protocols with higher dose of conventional radiotherapy, altered radiotherapy fractionation regimens, various boost techniques, neoadjuvant chemotherapy proved less efficacious. This study prospectively evaluated evidence for dose de-escalated Hypofractionated Radiotherapy with concurrent chemotherapy. Aims: To compare therapeutic effect, toxicity profile and quality of life parameter ‘Dysphagia’ using EORTC OES 18 questionnaire in study population. Settings and Design: Hospital Based Prospective Randomized Concurrent Parallel Open Labelled Two Arm Study. Methods and Material: Sixty eligible patients were enrolled to receive either conventional radiotherapy (CRT) or hypofractionated radiotherapy (HRT) on Cobalt-60 Unit. 30 patients received CRT with total dose of 63Gy/35 fractions for 7 weeks and 30 patients received HRT with total dose of 48 Gy/16fractions for 3 ½ weeks. Concurrent chemotherapy comprised of weekly cisplatin. Statistical analysis: Statistical software STATA 10.1 (2010), unpaired t-test, Paired t- test. Results: Both Arms in study were comparable in demographic parameters and Clinical parameters related to disease i.e. type of growth, length of lesion, TNM group staging. Improvement in weight, Overall survival and disease free survival were significantly better significantly better in CRT arm at follow up period of ≈1 ½ years. On Critical assessment of Quality of Life parameter ‘Dysphagia’, significantly better results were seen in CRT Arm. Patients requiring feeding gastrostomy/jejunostomy during treatment were significantly higher in HRT arm. However, overall (tumor) response was similar in both arms. Conclusion: This study evaluated feasibility of HRT in locally advanced cancer esophagus, results of the study indicate CRT arm being more effective as compared to HRT.
Keywords
Cancer esophagus; Conventional radiotherapy; Poor prognosis; Hypofractionated radiotherapy
Introduction
Cancer Esophagus (CE) is the eighth most common cancer worldwide, with an estimated 456,000 new cases in 2012 and the sixth most common cause of death with an estimated 400,000 deaths. CE has overall ratio of mortality to incidence of 0.88 [1]. Only 20% of the patients present with disease localized to esophagus, remaining having either locally advanced or metastatic disease. Concurrent chemo radiotherapy is standard of care for locally advanced cancer esophagus despite showing 50 % local failures. Survival rates of 25% to >35% at 5 years have been attained using array of treatment approaches however prognosis these patients remains poor despite recent advances in treatment delivery.
Protocols with higher dose of conventional radiotherapy, altered radiotherapy fractionation regimens, various boost techniques including brachytherapy, better chemotherapy molecules, neoadjuvant chemotherapy proved less efficacious.
Prospective study was done to explore whether locally advanced esophageal cancer is new frontier for Hypofractionated Radiotherapy.
Subjects and Methods
Primary objectives
• Overall response rate on the basis of Subjective and Objective parameters.
• Quality of life parameter “dysphagia” using EORTC OES 18 questionnaire.
Secondary objectives
• Disease free survival
• Overall survival
• Toxicity profile
Materials and Methods
It was a Hospital Based Prospective Randomized Concurrent Parallel Open Labelled Two Arm Study. After approval from Institutional Ethics Committee, 60 patients were enrolled in this study, 30 in each arm. Biopsy proven, non-metastatic (Stage I/ II/III) squamous cell carcinoma of middle 1/3 esophagus (as per anatomic classification) with ECOG performance status 0-2 and were included for study.
All patients underwent pre-treatment evaluation which included Medical history , physical examination, Complete blood count and biochemical profile, Routine chest X ray PA view, Barium swallow, Electrocardiography, Ultrasonography of abdomen, Computed tomography scan of Thorax, Upper GI endoscopy. Evaluation of clinical staging was based on CT scans as endoscopic ultrasound was unavailable [2]. The T stage was defined by the maximal transverse diameter of the esophageal tumor: T1 ≦ 2 cm, T2>2 cm and ≦4 cm, T3>4 cm. Tumors indicating an invasion of any adjacent structures were classified as T4. All patients provided written informed consent. Patients were treated by definitive chemo radiotherapy by 2D planning on Cobalt 60 (Theratron 780E) Unit. Both arms received concurrent chemotherapy (Inj. Cisplatin 30 mg/m2 weekly) during EBRT. Patients in arm A received five cycles whereas arm B patients were given two cycles.
Arm A received Conventional Fractionation i.e., EBRT 48.60 Gy in 27 fractions, 180cGy per fraction, 5 fractions in a week for total duration 5 ½ weeks while Arm B received Hypofractionated radiotherapy i.e. EBRT 36Gy in 12 fractions, 300 cGy per fraction, 5 fractions per week, total duration 2 ½ weeks.
EBRT Boost was given by three field technique (one anterior and two posterior oblique fields). Arm A was given 14.40 Gy in 8 fractions, 180 cGy per fraction, 5 fractions per week total duration 1 ½ week. Arm B receiving 12 Gy in 4 fraction of 300cGy for 1 week.
Overall (Tumor) response assessment in objective manner was performed by barium swallow, CECT Thorax and Upper GI Endoscopy at 1 month after completion of treatment. Patients were asked definite set of questions as per EORTC OES 18 Questionnaire to compare Quality of life parameter “Dysphagia”. Seven sub-scales were created to facilitate assessment. The monitoring of Toxicity was performed according to the Radiation Therapy Oncology Group criteria and the National Cancer Institute Common Terminology Criteria and Adverse Events (CTCAE) version 3.0.
Statistical Analysis
Statistical software STATA 10.1 (2010) was used for analysis. Continuous variables were presented as mean ± SD (standard deviation). Comparison within one arm, before and after intervention was compared using paired t- test while two arms were compared with each other using unpaired t- test. All Categorical variables compared by using Chi-Square test.
Kaplan- Meier survival curve was plotted to compare overall survival rate and disease free survival rate between two arms. Log rank test was performed to know significance of equality. All the tests were two sided. P value < 0.05 was considered as statistically significant.
Results
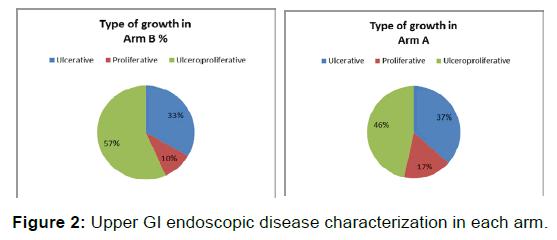
Mean Age in Arm A (60.7 ± 13.56 years) and Arm B (55.80 ± 12.42 years) were comparable. Gender wise distribution between two arms and Clinical parameters like type of growth was equally distributed in both the arms (p-value=0.658), NS [Figures 1 and 2 and Table 1].
| Arm A (CRT) | Arm B (HRT) | p-value | |||||
|---|---|---|---|---|---|---|---|
| Number | % | Number | % | ||||
| Demographic parameter | Age in years | < 40 | 2 | 6.67 | 3 | 10 | 0.381,NS |
| 40-65 | 16 | 53.33 | 20 | 66.67 | |||
| >65 | 12 | 40 | 7 | 23.33 | |||
| Gender | Male | 19 | 63.33 | 16 | 53.33 | 0.432,NS | |
| Female | 11 | 36.67 | 14 | 46.67 | |||
| Tumor and Treatment related parameters | TNM grouping | I-II | 12 | 40 | 8 | 26.67 | 0.273,NS |
| III | 18 | 60 | 22 | 46.67 | |||
| Radiotherapy | Regular | 21 | 70 | 16 | 53.33 | 0.184,NS | |
| Irregular | 9 | 30 | 14 | 46.67 | |||
| Chemotherapy | Complete | 21 | 70 | 20 | 66.67 | 0.781,NS | |
| Incomplete | 9 | 30 | 10 | 33.33 | |||
Table 1: Gender and age wise is distribution, tumor and treatment related parameters between two arms.
Length of lesion on barium swallow in Arm A was less than 5 cm in 11(36.67%) patients while 19 (63.33%) patients had length of lesion more than 5 cm. In Arm B, 13(43.33%) patients had length of lesion less than 5 cm on barium swallow while 17 (56.67%) patients had length of lesion more than 5 cm. Both the Arms were well matched over distribution of length of lesion (p-value=0.658), NS.
Tumor and treatment related factors like stage wise distribution, number of patients taking regular RT and intended chemotherapy cycles were similar between two arms [Figure 2 and Table 1].
Mean treatment duration in Arm A was 8.33 ± 1.82 weeks while in Arm B it was 5.76 ± 0.94 weeks making both Groups were well matched with respect to total intended treatment duration in each arms p-value=0.606, NS.
Results pertaining to subjective parameters like Swallowing capacity, improvement of weight and objective parameters like tumor response, loco regional failure, distant failure, disease status, and Survival status were shown in Table 2.
| Arm A (CRT) | Arm B (HRT) | p-value | |||||
|---|---|---|---|---|---|---|---|
| Mean | % | Mean | % | ||||
| Subjective parameters | |||||||
| Weight | Before treatment | 45.2 ± 10.86 | - | 42.57 ± 10.32 | - | 0.024, S | |
| After treatment | 47.33 ± 11.18 | - | 43.83 ± 10.10 | - | |||
| Improvement in swallowing capacity | Number | % | Number | % | |||
| Yes | 21 | 70 | 17 | 56.66 | 0.284,NS | ||
| No | 9 | 30 | 13 | 43.33 | |||
| Objective parameters | |||||||
| Tumor response | No residual disease | 21 | 70 | 20 | 66.67 | 0.781,NS | |
| Residual disease | 9 | 30 | 10 | 33.33 | |||
| Locoregional failure | Yes | 12 | 40 | 14 | 46.67 | 0.602,NS | |
| No | 18 | 60 | 16 | 53.33 | |||
| Distant failure | Yes | 6 | 20 | 7 | 23.33 | 0.754,NS | |
| No | 24 | 80 | 23 | 76.67 | |||
| Disease status | With disease | 16 | 53.33 | 17 | 56.67 | 0.754,NS | |
| Without disease | 14 | 46.67 | 13 | 43.33 | |||
| Survival status | Dead | 12 | 40 | 15 | 50 | 0.436,NS | |
| Alive | 18 | 60 | 15 | 50 | |||
Table 2: Subjective and objective parameters compared between two arms.
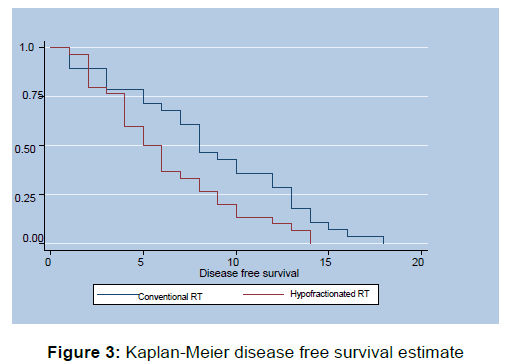
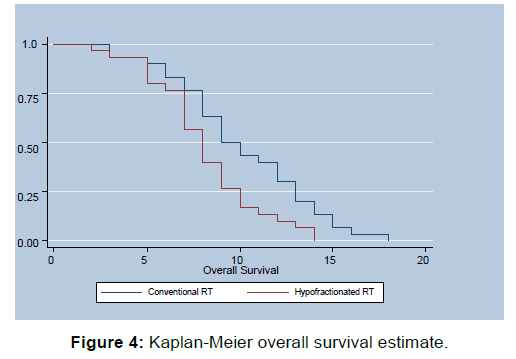
Quality of Life Assessment is summarized in Table 3, Whole questionnaire was compartmentalized into seven scales which were as follows, Dysphagia scale, Trouble swallowing saliva scale, Choking and coughing scale, Satisfaction scale, Dry mouth scale, esophageal reflux scale, Esophageal pain scale. Improvement of score in dysphagia scale was deemed as positive response while decreased score after intervention was deemed good response in other scales. Survival Analysis in the form of disease free survival (DFS) and Overall Survival (OS) was done utilizing Kaplan-Meier Method [Figures 3 and 4]. Assessment of toxicity parameters is depicted in Table 4.
| OES 18 Questionnaire Symptom score | Arm A | Arm B | Mean Diff | p-value | ||
| BT | AT | BT | AT | |||
| Dysphagia score | 9.3 ± 0.88 | 11.8 ± 1.90 | 9.33 ± 1.27 | 10.13 ± 1.50 | 1.7 | 0.001), HS |
| Trouble for swallowing saliva score | 2.77 ± 0.50 | 1.63 ± 0.81 | 2.77 ± 0.97 | 2.06 ± 1.05 | 1.07 | 0.001), HS |
| Choking & coughing score | 3.5 ± 0.78 | 3.2 ± 0.49 | 2.9 ± 0.71 | 2.5 ± 0.63 | 0.1 | 0.4986), NS |
| Satisfaction score | 12.33 ± 0.48 | 7.47 ± 2.39 | 10.3 ± 1.39 | 9.03 ± 1.83 | 3.59 | 0.001), HS |
| Dry mouth score | 3.1 ± 0.80 | 1.7 ± 0.75 | 2.87 ± 0.86 | 2.13 ± 1.13 | 0.66 | 0.0024), HS |
| Oesophageal reflux score | 4.8 ± 1.24 | 2.87 ± 0.68 | 5.3 ± 1.55 | 4.27 ± 1.93 | 0.9 | 0.0051), HS |
| Oesophageal pain score | 6.47 ± 1.57 | 4.5 ± 1.28 | 5.9 ± 1.24 | 4.73 ± 1.08 | 0.8 | 0.0113), HS |
Table 3: Various subscales of EORTC-OES18 questionnaire compared (BT- before treatment, AT- after treatment).
| Number | % | Number | % | p-value | |||
|---|---|---|---|---|---|---|---|
| Toxicity parameters | Nausea | Gr I | 19 | 63.33 | 20 | 66.67 | 0.787,NS |
| Gr II/III | 11 | 36.67 | 10 | 33.33 | |||
| Oesophagitis | Gr I/II | 22 | 73.34 | 6 | 20 | 0.001,S | |
| Gr III/IV | 8 | 26.66 | 24 | 80 | |||
| Stricture/ stenosis | Yes | 6 | 20 | 8 | 26.67 | 0.542,NS | |
| No | 24 | 80 | 22 | 73.33 | |||
| Feeding jejunostomy/ gastrostomy |
Yes | 2 | 6.67 | 9 | 30 | 0.02,S | |
| No | 28 | 93.33 | 21 | 70 |
Table 4: Acute and chronic toxicity parameters compared between two arms.
Discussion
The prognosis for patients with carcinoma of the esophagus remains poor despite recent advances in combined-modality therapies. At one hand definitive concurrent chemoradiotherapy achieved median survival and overall survival at 5 years on par with standard surgical management with better quality of life but at other end continued to show local failure as major cause of failure with approximately 50% of patients failing locally [3,4]. Median survival of 14 to 20 months and 5-year survival rates of 20% to 30% were achieved with chemoradiation alone [4-7]. These findings indicated that conventional fractionation scheme produced radiobiologically less tumoricidal effects for radioresistant CE [8]. Recent literature reported that radiation increased the expression of cancer stem cells markers for radiation resistance which could lead to the local failure [9-11]. Trials have indicated hypofractionated radiation offered a clear advantage over conventional radiation, especially in local control [9-12].
A HRT schedule delivers a dose larger than 2 Gy per fraction (with a lower overall dose). Hypofractionation can achieve improved therapeutic index in one of two ways when compared with the conventional fractionated scheme [13].: (i) dose escalation to increase tumour control (ii) maintaining dose equivalence in terms of tumour cure probability while decreasing the normal tissue dose. Number of other advantages is conferred in terms of logistical, patient convenience and resource allocation considerations [14]. Reduced numbers of fractions will reduce radiotherapy costs in terms of work-hours and fewer fractions also results in fewer visits which is more convenient and less costly [15].
Palliative EBRT (30Gy/10#) is also routine practice in advanced / metastatic disease to relieve dysphagia. HRT without concurrent chemotherapy has led to inferior outcome as compared to standard conventional fractionation [16,17]. Trials have shown that normal tissue toxicity is not increased during hypofractionated RT doses along with concurrent chemotherapy are if correct total dose adjustment is made [18-20]. Hence it is imperative to incorporate concurrent chemotherapy and dose de- escalated HRT to achieve optimum results along with minimum normal tissue toxicity.
In present study, we observed a male preponderance with a male to female ratio of 1.4:1, with majority of cases in the age group of 40-65 years which was in accordance with Indian studies exploring demographic profile of Oesophageal cancer [21-24]. Length of lesion on barium swallow is proven prognostic factor for survival in esophageal cancer [25,26]. This important prognostic factor was well matched between two treatment Arms. Upper GI Endoscopic disease characterization revealed either ulcerative, proliferative, ulcer proliferative growth. Both the Arms were comparable with respect to distribution of tumor growth characterization.
Though non-significant, but there was better trend in adherence to treatment protocol in conventional fractionation arm as evidenced by 70% of patients taking treatment regularly in Arm A Vs 53.33% patients in Arm B. Both Arms were well matched with respect to patient completing intended treatment in stipulated duration (p-value=0.606), NS.
Improvement in weight in CRT Arm was statistically better than results observed in HRT Arm. Positive change in swallowing capacity was comparable in both arms (p-value=0.284), NS, but better trend of this change was improved in HRT Arm. This can be attributed to proven effect of higher dose RT for relieving dysphagia which is commonly used in Palliative setting.
Objective response assessment was done using Barium swallow, CECT Thorax and Upper GI Endoscopy. In case of discrepancy of results, findings of Upper GI Endoscopy were considered benchmark since after resection or radiotherapy for esophageal carcinoma, the normal soft tissue planes in the mediastinum are altered or obliterated and recurrent/residual tumor can be very difficult to identify in this setting [27]. Overall tumor responsein the study was comparable (p-value=0.781), NS. Both the Arms were comparable for locoregional failure (40% vs 46.67%) and distant failure (20% vs 23.33) with p-value of 0.602 and 0.754 respectively.
Since last two decades there is growing interestin broadening the evaluation criteria employed in cancer clinical trials i.e., Quality of life (QOL) during the limited survival time. Dysphagia has an overwhelming influence on patient’s quality of life, hence in this study we assessed dysphagia related parameter, using EORTC OES-18 module. Literature review pertaining to quality of life in esophageal cancer patients undergoing concurrent chemoradiotherapy is scarce. Analysis between two Arms revealed, except choking and coughing scale all other scales showed statistically significant improvement for CRT (Arm A) as compared to HRT (Arm B).
Median DFS was 8 months for conventional RT, while 5.5 months for Hypofractinated RT Arm. Arm A showed statistically significant Better DFS as compared to Arm B Log rank value=0.135, (p-value=0.001),HS
Median Overall Survival in Arm A was 9.5 months while for Arm B it was 8 months. Overall Survival Analysis generated utilizing Kaplan-Meier Method showed statistically significant better OS for Arm A as compared to Arm B Log rank value- 0.110, (p-value=0.001), HS.
These results were contradictory to study done by Ma JB [28]. 3- and 5-year survival rates (43.2% and 38.8%, 38.2% and 28.0%, respectively) were not significantly different between the Hypofractionated and Conventional schemes (P=0.268). This can be attributed to limited follow up period of 1½ years, utilization of different chemotherapy molecule and adjuvant chemotherapy post concurrent chemoradiotherapy in reference study.
Feeding Gastrostomy/ Jejunostomy placement, which correlated with acute toxicity of regimen was significantly higher in Hypofractionated RT Arm as compared to conventional Arm (p-value=0.02).
In both Arms no patient had late toxicity in form of radiation pneumonitis, stricture/stenosis.
Conclusion
Hypofractionation in Cancer Esophagus is the need of the day because of advanced stage of disease at presentation, need of palliative treatment in most of the cases and better resource utilization. Even though, this study showed better results with respect to Quality of Life, Disease Free Survival, Overall Survival, and Toxicity Profile for Conventional Radiotherapy as compared to Hypofractionated Radiotherapy, overall (tumor) response was similar in both Arms. Hence it will be worthwhile to see the results of Hypofractionated treatment protocol in Cancer esophagus with large number of patients, longer follow up and modern radiation delivery techniques which may truly establish the role of Hypofractionated Radiotherapy. Till then concurrent chemoradiation should be standard of care in locally advanced middle third cancer esophagus.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Oesophageal Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012 Retrieved from http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Brown LM, Swanson CA, Gridley G, et al. Adenocarcinoma of the esophagus: role of obesity and diet. J Natl Cancer Inst. 1995;87:104-109.
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593-1598.
- Al-Sarraf M, Martz K, Herskovic A, et al. Progress report of combined chemoradiotherapy versus radiotherapy alone in patients with esophageal cancer: An intergroup study. J Clin Oncol.1997;15:277-284.
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623-1627.
- Araujo CM, Souhami L, Gil RA, et al. A randomized trial comparing radiation therapy versus concomitant radiation therapy and chemotherapy in carcinoma of the thoracic esophagus. Cancer.1991;67:2258-2261.
- Roussell A, Jacob J, Haegele P, et al. Controlled clinical trial for the treatment of patients with inoperable esophageal carcinoma: a study of EORTC Gastrointestinal Tract Cancer Cooperative Group. Rec Results Cancer Res. 1988;110-121.
- Halperin EC, Perez CA, Brady LW, et al (2008). Biologic Basis of Radiation Therapy. In: McBride W.H. and Withers H.R. (eds.), Perez and Brady’s principles and practice of radiation oncology. Lippincott Williams and Wilkins, Lippincott Williams and Wilkins, pp. 77-109.
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature.2006;444:756-760.
- Hermann PC, Huber SL, Herrler T, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell.2007;1: 313-323.
- Nguyen GH, Murph MM, Chang J Y. Cancer Stem Cell Radioresistance and Enrichment: Where Frontline Radiation Therapy May Fail in Lung and Esophageal Cancers. Cancers (Basel).2011;3:1232-1252.
- Sykes AJ, Burt PA, Slevin NJ, et al. Radical radiotherapy for carcinoma of the oesophagus: an effective alternative to surgery. Radiother Oncol.1998;48:15-21.
- Ritter M. Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin Radiat Oncol. 2008;18:249-256.
- Parthan A., Pruttivarasin N., Davies D. Comparative cost-effectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer. Frontiers Oncol. 2012;2:81.
- Sethukavalan P, Cheung P, Tang CI. Patient costs associated with external beam radiotherapy treatment for localized prostate cancer: the benefits of hypofractionated over conventionally fractionated radiotherapy. Can J Urol. 2012;19:6165-6169.
- Launois B, Delarue D, Campion JP, et al. Preoperative radiotherapy for carcinoma of the esophagus. Surg Gynecol Obstet 1981;153:690-692
- Gignoux M, Roussel A, Paillot B, et al. The value of preoperative radiotherapy in esophageal cancer: results of a study of the E.O.R.T.C. World J Surg 1987;11:426-432.
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-467.
- Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161-167.
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-668.
- Sankaranarayanan R. Risk factors for cancer of the oesophagus in Kerala, India. Int J Cancer. 1991;49:485-489.
- Sehgal S, Kaul S, Gupta BB, Dhar MK. Risk factors and survival analysis of the esophageal cancer in the population of Jammu, India. Indian J Cancer. 2012;49:245-250.
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150.
- Giri PA, Singh KK, Phalke DB. Study of socio-demographic determinants of esophageal cancer at a tertiary care teaching hospital of Western Maharashtra, India. South Asian Journal of Cancer. 2014;3:54-56.
- Chen C.Z., et al. Long-term outcomes and prognostic factors for patients with esophageal cancer following radiotherapy. World Journal of Gastroenterology : WJG. 2013;19, 1639-1644.
- Yendamuri S, et al. Esophageal tumor length is independently associated with long-term survival. Cancer. 2009;115:508-516.
- Vaishnav, Kavita U., et al. "Role of CT Scan in staging of carcinoma of esophagus–A study of 100 Cases." Chest 26: 26.
- Ma JB. Moderately hypofractionated conformal radiation treatment of thoracic esophageal carcinoma. Asian Pac J Cancer Prev. 2012;13:4163-4167.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.