Fixed Dose Combination of Arterolane and Piperaquine: A Newer Prospect in Antimalarial Therapy
- *Corresponding Author:
- Dr. Chetan Yuvraj Patil
Department of Pharmacology, Government Medical College and Hospital, Aurangabad ? 431 001, Maharashtra, India.
E-mail: drchetanpatil.gmc@gmail. com
Abstract
Malaria has been very prevalent vector borne disease in India and until date bears enormous implications on health care services of the country. Over the period of time, the development of resistance to traditional antimalarials like chloroquine has been posed as major deterrent in efforts of malaria control. As the drug resistance is today universally prevalent, especially in Plasmodium falciparum species, major burden of malarial control resides with the new artemisinin drug class. However, arterolane is one of the first fully synthetic non-artemisinin antimalarial compound with rapid schizontocidal activity, hence offering an alternative to artemisinin drugs in malaria control. Piperaquine is a synthetic bisquinoline (4-amioquinoline Antimalarial) with slow and longer schizontocidal activity. Therefore their combination has been shown to provide rapid parasitemic clearance and quick relief of most malaria related symptoms along with prevention of recrudescences. This combination was approved by Drugs Controller General of India in 2011 for treatment of uncomplicated P. falciparum malaria. The article is aimed at to review this newer prospect in antimalarial therapy for which comprehensive database search was done in Google, Google Scholar, PubMed using the terms “Malaria,” “Arterolane,” “OZ277,” “Piperaquine,” and “Artemisinin combination therapy.” A total of 323 articles were screened and 28 articles were considered for this review along with the World Health Organization and National malarial program guidelines.
Keywords
Arterolane, Malaria, Piperaquine
Introduction
World Malaria Report 2011 of World Health Organization (WHO) documents the global malarial burden of 216 million cases in year 2010, out of which South-East Asian region accounts for 13% of the cases.[1] India shares an enormous 75% of the 2.5 million reported malaria cases in South-East Asia. More than 60% of the Indian population resides in the malaria-affected parts of the country.[2,3] WHO documents 15,000 annual malaria-related deaths in India, including significant pediatric victims, which seems to be conservative figure compared with staggering 205,000 [1] annual death toll cited in certain journals.[4,5]
Traditionally all malarial species could be treated and prevented with available drug classes of quinolines (primarily chloroquine, mefloquine, quinine), sulfonamides (sulfadoxinepyrimethamine) and tetracyclines.[6] Over the period of time, the malarial parasite has progressively acquired marked resistance to available drugs like chloroquine and sulphadoxine-pyrimethamine making them clinically ineffective against most species notably Plasmodium falciparum followed by Plasmodium vivax.[6] In fact chloroquine resistance is now universally prevalent in P. falciparum species.[7] Discovery of antimalarial efficacy of artemisinin derivatives even against species resistant to traditional drugs has placed a major burden of malarial control on this drug class.[8] A definitive scope for discovery of newer non-artemisinin drugs effective against resistant parasites prevails.
Further, the preliminary reports of reduced efficacy of artemisinin therapy in P. falciparum parasite in certain parts of the world,[9] could prove deterrent to the current efforts of malaria control. Development of such drug resistance in parasites can be prevented if antimalarials are administered as a combination therapy.[10] WHO therefore recommends use of two antimalarials simultaneously, especially when the antimalarials have different mechanisms of actions, that would provide potential for inhibition of development of resistance to either of the component when used alone.[6,11]
The need for newer artemisinin-like fast acting effective antimalarial led to development of a fully synthetic non-artemisinin ozonide compound OZ277 (Arterolane). This compound exhibited antimalarial action against all asexual blood stages of P. falciparum with a rapid onset of action in established murine model of malaria. In phase II trials, arterolane monotherapy showed insufficient activity in patients owing to decreased exposure levels as compared to healthy volunteers. However, further clinical development in combination with piperaquine showed higher blood levels as well as high clinical activity, with patients being free from recrudescences after therapy.[12,13] Therefore, a newer fixed dose combination (FDC) of arterolane and piperaquine seeks to meet the dual requirement of an effective non-artemisinin antimalarial agent (arterolane) that is administered as combination (with piperaquine) in accordance to WHO recommendation to prevent further parasite resistance. This FDC of arterolane-piperaquine has been approved by Drug Controller General of India (DCGI) in 2011 in treatment of P. falciparum malaria.[14]
Methods of Literature Search
The literature for this review was searched for full text and abstracts from the various indices like Google, Google Scholar, PubMed and Medline using the keywords “Malaria,” “Arterolane,” “OZ277,” “Piperaquine,” and “Artemisinin combination therapy.” A total number of 323 articles were found and screened for reviews and randomized controlled trials of arterolane, OZ277 and piperaquine in last 20 years. Of these, 28 articles were considered for this review. The background information of malarial epidemiology and the current scenario of artemisinin based therapy was obtained from WHO reports and recent reviews found in PubMed and Google Scholar. The current guidelines for malaria control as per the WHO and National malaria control program of India were considered.
FDC (Arterolane + Piperaquine)
The FDC consists of two parasiticidal drug namely rapidly acting arterolane and longer acting piperaquine. The combination has been approved by the DCGI in 2011.[14]
Mode of Action
Arterolane
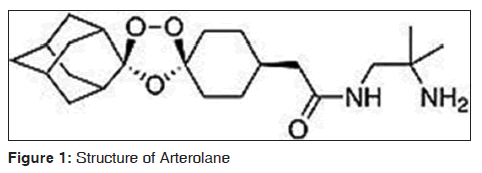
Arterolane is one of first fully synthetic trioxolane peroxide, non-artemisinin antimalarial compound.[15] The molecular formula is C26H40N2O8 and molecular weight is 508.61. The Structural formula as shown in Figure 1.[16-18]
It has rapid schizontocidal activity against all erythrocytic stages of P. falciparum without any effect on hepatic stages.[15] This action of arterolane is attributed to inhibition of heme detoxification and Pf-encoded sarcoplasmic endoplasmic reticulum calcium ATPase (PfATP6). Arterolane is an active moiety, which gets accumulated either in cytosol or food vacuole of the parasite.[17,19] In the food vacuole, the peroxide bond of arterolane undergoes reductive cleavage by ferrous iron (Fenton reaction). The resulting free radicals produced from this irreversible redox reaction cause alkylation of varied protein targets viz. endoplasmic reticulum membrane associated PfATP6 resulting in inhibition of ATP-dependent Ca+2 pump, which is homologous to mammalian sarcoplasmic/endoplasmic reticulum Ca2+ ATPase. The reactive radicals are proposed to subsequently cause prevention of heme detoxification by reacting with iron-bound ferriprotoporphyrin IX itself and also inhibit a multitude of intracellular enzymes.[15,17,19]
Piperaquine
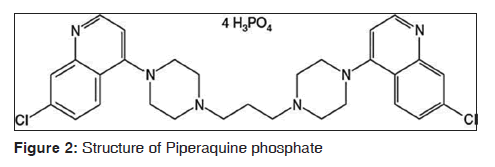
Piperaquine a synthetic bisquinoline compound belonging to 4-amioquinoline group of antimalarials. The molecular formula of piperaquine phosphate is C29H32C12N6.4H3PO4.4H2O and molecular weight is 999.56.[20] The structural formula is given in Figure 2.[20]
Piperaquine has a slow and longer schizontocidal activity against erythrocytic stages of both P. vivax and P. falciparum and chloroquine-resistant plasmodium strains.[21-23] Most evidences conclusively propose inhibition of parasite heme-digestion pathway, similar to action of chloroquine.[24,25] Failure to inactivate heme and toxicity of drug-heme complexes is thought to kill the parasites via oxidative damage to membranes, digestive proteases or other critical biomolecules such as nucleic acid, phospholipids etc., A good activity against chloroquine-resistant strains may be attributed to bulky bisquinoline structure itself, which possibly also contributes to inhibition of chloroquine efflux transporters from the parasite food vacuole.[21-23]
Therefore the combination of short acting arterolane with long-acting piperaquine potentially offers rapid parasitemic clearance and quick relief of most malaria-related symptoms along with prevention of recrudescences. The FDC formulation provides an alternative complementary combination therapy to ACTs as per WHO recommendations
Indications
The FDC is indicated in treatment of acute, uncomplicated P. falciparum malarial infection in individuals from 12 to 65 years.[15] For the age group <12 and >65 years the antimalarial treatment can be administered as recommended by WHO.[6] It has been found to be effective in geographical regions with reported chloroquine resistance. However, it is not indicated for patients with severe or complicated P. falciparum malaria or for prevention of malaria.[15,26]
Dosing schedule
Each tablet of FDC consists of arterolane (150 mg) and piperaquine (750 mg). The recommended regimen is a 3-dose treatment schedule given as single dose on diagnosis, followed with subsequent doses at 24 and 48 h after first dose.[15,27]
The FDC can be taken with or without food, though administration with food shows improved absorption of arterolane and piperaquine. A repeat dose needs to be taken, in case of vomiting within 1 h of administration.[15,26,27]
Pharmacokinetics
Arterolane
Arterolane is well absorbed on oral administration. The peak plasma concentrations are attained at (Tmax) 4.5-5.25 h in healthy young male subjects. Secondary peaks in plasma concentration may be attributed to variable regional gut absorption, gastric emptying or enterohepatic cycling. Given with food, more the one-third increase in systemic exposure is observed. Study in healthy Thai subjects illustrates no significant influence of dosing time, gender or ethnicity on pharmacokinetics. At concentration range of 200 and 2000 ng/mL in humans, arterolane has ~ 93% plasma protein binding and wide volume of distribution. It is majorly metabolized by CYP3A4-catalyzed oxidation of adamantane moiety of arterolane producing a fairly stable compound in human hepatocytes, though CYP3A4 is unlikely to play a major role in the systemic clearance. Arterolane though has rapid elimination rate with short half-life of 2-4 h.[15,26-28]
Piperaquine
Piperaquine too has good absorption on oral administration with average Tmax of 2.5-4.5 h.[29] Presence of food doubles the systemic exposure of piperaquine compared with when administered alone. The observations from pharmacokinetic studies are consistent with the long elimination half-life relative to the dosing interval with mean terminal half-lives ranging from 11-18 days and 17-23 days following single dose and multiple dose administration respectively.[25] At concentration ranges of 100-1000 ng/ml in humans, piperaquine is highly bound (>99%) to plasma proteins but not to human red blood cells and has an extensive volume of distribution.[15,28]
The microsomal isoenzyme CYP3A4 is primarily responsible for the Phase I metabolism of piperaquine. Five major metabolites were detected in urine namely, carboxylic metabolite (M1), N-oxidated metabolite (M2) and hydroxylated metabolite (M3 to M5). Enterohepatic recycling of Piperaquine is indicated in consideration with its high molecular weight and pharmacokinetic properties.[30,31]
Arterolane-piperaquine combination
Following single dose administration, pharmacokinetic parameters of FDC in young fasting healthy male subjects depicts in Table 1.[15]
| Parameter | Arterolane | Piperaquine |
|---|---|---|
| Tmax (h) | 3.65 | 5.13 |
| Cmax (ng/mL) | 43.97 | 83.42 |
| AUC0-24 (ng.h/mL) | 299.56 | 837.43 |
| t1/2 | 2.44 h | 15 days |
FDC tablet of Arterolane 150 mg and Piperaquine phosphate 750 mg. AUC0-24: Area under plasma concentration-time curves in first 24 h of drug administration, Tmax: Time required to attained peak plasma concentrations, Cmax: Maximum plasma concentrations, t1/2: Half-life, FDC: Fixed dose combination
Table 1: Pharmacokinetic parameters of FDC
Since CYP3A4 has been indicated in primary metabolism of both arterolane and piperaquine the combination was tested for any possible metabolic interaction. However, inhibitory action of arterolane occurs at higher concentrations and is unlikely to affect metabolism at therapeutically used doses. In comparison, piperaquine being a CYP3A4 inhibitor, can reduce arterolane metabolism.[15,18,27]
FDC in Preclinical and Clinical Studies
The preclinical studies in efficacy models of P. falciparum malaria using Swiss mice infected with rodent specific species Plasmodium berghei, indicates it to be more potent than the standard reference antimalarial drugs-chloroquine, mefloquine and artesunate.
Piperaquine’ antimalarial activity examined against chloroquine in P. berghei strains in mice demonstrates good in vivo activity, significantly lower in vitro resistance index and considerable activity against chloroquine resistance strains in mice, without a significant toxicity. Using model of P. berghei-infected mice, experimental in vivo testing of the combination with different doses and ratios of each compound concluded an additive action between arterolane and piperaquine.[32]
Clinical studies carried out using the arterolane-piperaquine combination in acute uncomplicated P. falciparum malaria patients provide evidence of high clinical efficacy evaluated on indicative parameters of adequate clinical and parasitological response (ACPR), fever clearance time (FCT) and parasite clearance time (PCT). Studies show high cure rate attained in Malaria Endemic countries with widespread Chloroquine-resistance, with confirmed efficacy in comparative study conducted in India, Thailand and Bangladesh.[33]
In phase III study, A randomized, double-blind, multicenter comparative trial with artemether-lumefantrine in acute uncomplicated P. falciparum malaria, revealed the FDC to have shorter FCT (18 h vs. 24 h) and equivalent PCT (36 h vs. 34 h), cure rates at day 28 (97.9% vs. 98.9%, each meeting WHO criteria of >95%) and gametocidal activity in comparison to artemether-lumefantrine.[34]
In phase II study, a randomized, open-label, multicenter trial, on other hand observed 28 day polymerase chain reaction corrected ACPR to be 100% with arterolane-piperaquine against 98.7% with artemether-lumefantrine in per protocol population and identical median in PCT and FCT.[15,34]
Safety Profile
Safety of arterolane-piperaquine combination as evaluated by its exposure shows the combination to be well-tolerated in adult patients of uncomplicated falciparum malaria. the reported clinical adverse events mostly include headache and gastrointestinal side effects. These adverse events were mostly mild, resolving without any squeal. The most common clinical adverse events reported was anemia, which was not significant when compared to other treatment regimen. Other adverse events reported were headache, nausea, vomiting, anorexia, dyspepsia, abdominal pain, anorexia, cough, dizziness.[15,28]
Precautions
Arterolane-piperaquine combination has been shown to affect QTc interval. Trials have shown QTcF >500 ms in about 1% of patients and change in QTcF interval >60 ms from baseline in about 5% of patients.[15] However, QTc intervals were in normal range for subjects treated with arterolane and piperaquine alone.[20,26,35]
Therefore caution is recommended in patients of:[36]
• Prolonged QT interval (due to either congenital disorders like long QT syndrome or acquired clinical condition like symptomatic cardiac arrhythmias or with severe cardiac disease)
• Family history of congenital QT interval prolongation
• Electrolyte disturbances e.g., hypokalemia, hypomagnesaemia
• Concomitant medication with drugs known to prolong the QT interval such as class IA and class III antiarrthymic agents (quinidine, procainamide, disopyramide and amiodarone, sotalol); antipsychotics (pimozide, ziprasidone); antidepressants; certain antibiotics (macrolides, fluoroquinolones, imidazole and triazole antifungal agents); certain antihistaminics (terfenadine, astemizole), or cisapride.
Contraindications
Hypersensitivity to any of component of drug combination is an absolute contraindication. Currently, No other specific contraindication are known.[15,26,28]
Overdose
Higher levels of exposure of arterolane and piperaquine, tested at single doses of 600 mg and 1500 mg respectively have shown to be tolerated well in humans. No formal data is available for overdose of the combination. In suspected cases of over-dosage, appropriate symptomatic and supportive management, including electrocardiogram and blood electrolyte monitoring is recommended.[25,26]
Drug interactions
Since CYP3A4 is the primary metabolizing isoenzyme for both arterolane and piperaquine, any co-administered drug having known designs of inhibition, induction or competition for CYP3A4 can potentially display pharmacokinetic interaction with the antimalarial combination. Anti-retroviral drugs, such as protease inhibitors and non-nucleoside reverse transcriptase inhibitors, owing to varied patterns of effects namely increase or decrease in activity of microsomal CYP34A enzyme system, shows unpredictable effect on systemic arterolane and piperaquine concentrations. This may result in toxic effect, such as prolonged QT interval or loss of antimalarial efficacy depending on serum concentration attained. Hence caution need to be exercised when administered with Anti-Retroviral drugs.[15,28,37]
Due to potential effect of QT interval prolongation with arterolane-piperaquine, the combination may exhibit a pharmacodynamic interaction with drugs with potential additive effect on QT interval prolongation. These include other antimalarials such as Quinine, Quinidine and halofantrine and thus should be avoided within 3 months of each other owing to long elimination half-life of piperaquine. Clinical drug interaction studies have not been performed for the arterolane-piperaquine combination.[36]
Pediatrics and geriatrics
As the clinical studies of arterolane-piperaquine in uncomplicated P. falciparum malaria excluded pediatric patients less than 12 years and geriatric patients aged above 65 years, the safety and efficacy of this combination remains unestablished in this patient subgroup. Therefore, the presence of concomitant disease, drug therapy and status of hepatic, renal and cardiac function should be considered before prescribing arterolane-piperaquine in this subgroup of patients.[15,26,27]
Hepatic and renal impairment
The pharmacokinetic data on metabolism of arterolane and piperaquine shows that primary hepatic clearance does not play major role in systemic clearance and that very little of drug is excreted unchanged in urine.[15,26,27,30] Several patients of acute malaria usually suffer some degree of hepatic and/or renal impairment and studies have shown arterolane-piperaquine at therapeutic doses of 150-750 mg respectively exhibit no significant adverse event or loss of efficacy. Precaution is therefore recommended for administration of arterolane-piperaquine combination in patients with moderate to severe hepatic or renal impairment.[38]
Conclusion
FDC of rapidly and short acting arterolane and slow and long acting piperaquine eliminates the residual parasites. It also provides a simplified once-a-day dosing 3 day therapy for the treatment of acute uncomplicated P. falciparum malaria in adults. This convenient short course treatment may also encourage compliance. This endows an alternative therapy in malaria control with potential role in treatment of resistant malaria and prevention of further development of parasite resistance.
References
- World Health Organization. World Malaria Report 2011. Geneva: World Health Organization; 2008. Available from: http://www.apps.who.int/iris/bitstream/10665/44792 /2/9789241564403_eng_full.pdf. [Last accessed on 2013 Jan 05].
- Dash AP, Valecha N, Anvikar AR, Kumar A. Malaria in India: Challenges and opportunities. J Biosci 2008;33:583-92.
- A Profile of National Institute of Malaria Research. Estimation of True Malaria Burden in India. p. 91-9. Available from: http://www.mrcindia.org/MRC_profile/profile2/ Estimation of true malaria burden in India.pdf. [Last accessed on 2013 Jan 5].
- Dhingra N, Jha P, Sharma VP, Cohen AA, Jotkar RM, Rodriguez PS, et al. Adult and child malaria mortality in India: A nationally representative mortality survey. Lancet 2010;376:1768-74.
- Hay SI, Gething PW, Snow RW. India’s invisible malaria burden. Lancet 2010;376:1716-7.
- Bloland PB. Drug Resistance in Malaria. Document WHO/ CDS/CSR/DRS/2001.4. Geneva: World Health Organization; 2001. Available from: http://www.who.int/csr/resources/ publications/drugresist/malaria.pdf. [Last accessed on 2009 May 14].
- Setthaudom C, Tan-ariya P, Sitthichot N, Khositnithikul R, Suwandittakul N, Leelayoova S, et al. Role of Plasmodium falciparum chloroquine resistance transporter and multidrug resistance 1 genes on in vitro chloroquine resistance in isolates of Plasmodium falciparum from Thailand. Am J Trop Med Hyg 2011;85:606-11.
- Bhattarai A, Ali AS, Kachur SP, Mårtensson A, Abbas AK, Khatib R, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med 2007;4:e309.
- Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009;361:455-67.
- Nosten F, Brasseur P. Combination therapy for malaria: The way forward? Drugs 2002;62:1315-29.
- World Health Organization. WHO Briefing on Malaria Treatment Guidelines and Artemisinin Monotherapies. Geneva: World Health Organization; 2006. Available from: http://www.who.int/malaria/publications/atoz/meeting_ briefing19april.pdf. [Last accessed on 2013 Jan 15].
- Charman SA, Arbe-Barnes S, Bathurst IC, Brun R, Campbell M, Charman WN, et al. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc Natl Acad Sci U S A 2011;108:4400-5.
- Olliaro P, Wells TN. The global portfolio of new antimalarial medicines under development. Clin Pharmacol Ther 2009;85:584-95.
- Central Drugs Standard Control Organization. List of Drug Approved for Marketing in India 2011. Available from: http://www.cdsco.nic.in/NEW%20DRUG%20UPDATE. doc. [Last accessed on 2012 Jan 9].
- Valecha N, Krudsood S, Tangpukdee N, Mohanty S, Sharma SK, Tyagi PK, et al. Arterolane maleate plus piperaquine phosphate for treatment of uncomplicated Plasmodium falciparum malaria: A comparative, multicenter, randomized clinical trial. Clin Infect Dis 2012;55:663-71.
- Kaiser M, Wittlin S, Nehrbass-Stuedli A, Dong Y, Wang X, Hemphill A, et al. Peroxide bond-dependent antiplasmodial specificity of artemisinin and OZ277 (RB×11160). Antimicrob Agents Chemother 2007;51:2991-3.
- Fügi MA, Wittlin S, Dong Y, Vennerstrom JL. Probing the antimalarial mechanism of artemisinin and OZ277 (arterolane) with nonperoxidic isosteres and nitroxyl radicals. Antimicrob Agents Chemother 2010;54:1042-6.
- Maerki S, Brun R, Charman SA, Dorn A, Matile H, Wittlin S. In vitro assessment of the pharmacodynamic properties and the partitioning of OZ277/RBx-11160 in cultures of Plasmodium falciparum. J Antimicrob Chemother 2006;58:52-8.
- Uhlemann AC, Wittlin S, Matile H, Bustamante LY, Krishna S. Mechanism of antimalarial action of the synthetic trioxolane RBX11160 (OZ277). Antimicrob Agents Chemother 2007;51:667-72.
- Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: A resurgent antimalarial drug. Drugs 2005;65:75-87.
- Raynes K. Bisquinoline antimalarials: Their role in malaria chemotherapy. Int J Parasitol 1999;29:367-79.
- Raynes K, Foley M, Tilley L, Deady LW. Novel bisquinoline antimalarials. Synthesis, antimalarial activity, and inhibition of haem polymerisation. Biochem Pharmacol 1996;52:551-9.
- Vennerstrom JL, Ellis WY, Ager AL Jr, Andersen SL, Gerena L, Milhous WK. Bisquinolines. 1. N, N-bis (7-chloroquinolin-4-yl) alkanediamines with potential against chloroquine-resistant malaria. J Med Chem 1992;35:2129-34.
- O’Neill PM, Bray PG, Hawley SR, Ward SA, Park BK. 4-Aminoquinolines – Past, present, and future: A chemical perspective. Pharmacol Ther 1998;77:29-58.
- Ahmed T, Sharma P, Gautam A, Varshney B, Kothari M, Ganguly S, et al. Safety, tolerability, and single- and multiple-dose pharmacokinetics of piperaquine phosphate in healthy subjects. J Clin Pharmacol 2008;48:166-75.
- Valecha N, Looareesuwan S, Martensson A, Abdulla SM, Krudsood S, Tangpukdee N, et al. Arterolane, a new synthetic trioxolane for treatment of uncomplicated Plasmodium falciparum malaria: A phase II, multicenter, randomized, dose-finding clinical trial. Clin Infect Dis 2010;51:684-91.
- Gautam A, Ahmed T, Sharma P, Varshney B, Kothari M, Saha N, et al. Pharmacokinetics and pharmacodynamics of arterolane maleate following multiple oral doses in adult patients with P. falciparum malaria. J Clin Pharmacol 2011;51:1519-28.
- Kumar RR, Velvizhy R. Newer artemisinin combination therapy for uncomplicated malaria. Phisci 2012;3. Available from: http://www.Phisci.In/Journal/Index.Php/Phisci/ Article/View/101. [Last accessed on 2013 Mar 23].
- Liu C, Zhang R, Hong X, Huang T, Mi S, Wang N. Pharmacokinetics of piperaquine after single and multiple oral administrations in healthy volunteers. Yakugaku Zasshi 2007;127:1709-14.
- Chen Q, Deng J, Wu D. Study on absorption, distribution and excretion of 14C-piperaquine phosphate and 14C-piperaquine in mice. Pharm Ind 1979;8:19-23.
- Tarning J, Bergqvist Y, Day NP, Bergquist J, Arvidsson B, White NJ, et al. Characterization of human urinary metabolites of the antimalarial piperaquine. Drug Metab Dispos 2006;34:2011-9.
- Snyder C, Chollet J, Santo-Tomas J, Scheurer C, Wittlin S. In vitro and in vivo interaction of synthetic peroxide RBx11160 (OZ277) with piperaquine in Plasmodium models. Exp Parasitol 2007;115:296-300.
- Aditya S, Nandha R, Sekhri K, Tyagi S. Arterolane maleate and piperaquine phosphate: A new option in the treatment of Plasmodium falciparum malaria. J Drug Discov Ther 2013;1:66-9.
- Ranbaxy Laboratories Limited Efficacy Synriam™. Available from: http://www.synriam.com/about-synriam/ efficacy/. [Last accessed on 2013 Jun 15].
- Karunajeewa H, Lim C, Hung TY, Ilett KF, Denis MB, Socheat D, et al. Safety evaluation of fixed combination piperaquine plus dihydroartemisinin (Artekin) in Cambodian children and adults with malaria. Br J Clin Pharmacol 2004;57:93-9.
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med 2004;350:1013-22.
- Dooley KE, Flexner C, Andrade AS. Drug interactions involving combination antiretroviral therapy and other anti-infective agents: Repercussions for resource-limited countries. J Infect Dis 2008;198:948-61.
- Sharma SK, Sharma BH, Shakya K, Khanal B, Khaniya S, Shrestha N, et al. Acute renal failure and hepatic dysfunction in malaria. J Nepal Med Assoc 2004;43:7-9.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.