Formulation and Evaluation of Bioadhesive Vaginal Tablet of Tamarind Gum Containing Acyclovir
Received: 14-Jun-2021 Accepted Date: Jun 21, 2021 ; Published: 26-Nov-2021
Citation: Hemant KS Yadav et al. Formulation and Evaluation of Bioadhesive Vaginal Tablet of Tamarind Gum Containing Acyclovir. Ann Med Health Sci Res.2021;11:176-180
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
The aim of the study was to formulate bio adhesive vaginal tablets using natural tamarind gum. Acyclovir was chosen as a model drug. Tamarind gum was extracted from the seeds of tamarind fruit. Tablets were compressed by direct compression. Compatibility studies were done for the gum and drug. FTIR and DSC studies showed that there was no chemical interaction between the gum and the drug. All the prepared formulation showed satisfactory quality control parameters. Tablets showed good bio adhesion. It can be concluded that the prepared tablets can be effectively used as vaginal drug delivery system.
Keywords
Tamarind; Acyclovir; Bio adhesion; Vaginal
Introduction
There are various conventional vaginal drug delivery systems which are available in form of foams, creams and gels. The limitations of these systems are that they exhibit leakage, residence time in the site of application is less and they are difficult to handle as a result patient compliance is poor. [1]
Compared to these dosage forms vaginal tablets are more useful dosage forms as they are easy to use and any dose of drug can be easily incorporated. [2] Research has been done in preparing multilayered vaginal tablets for administration of antiretroviral drug. [3]
The use of mucoadhesive polymers can help in increasing the residence time at the site of application. Tamarind gum has been used to prepare matrix tablets. [4] In the current study tamarind gum was used as mucoadhesive polymer for the preparation of vaginal tablets. Tamarind gum, also called Tamarind Seed Polysaccharide (TSP for short), is a kind of neutral polysaccharide substance extracted and separated from endosperm of legume tamarind plant seeds. [5]
Tamarind gum has many applications in food, paper and pharmaceuticals. From the pharmaceutical point of view it helps to improve the dissolution rate of poorly water-soluble drugs and has the property to enhance the mucoadhesion. Tamarind gum has high molecular weight 52350 units and monomer of glucose, galactose and xylose in molar ratio of 3:1:2. Thus, It can be also used as suitable polymer for sustained release formulations for controlled release of both water-soluble and water insoluble drugs. [6]
Tamarind Gum (TG) is a plant polysaccharide extracted from seed endosperm of the plant, Tamarindus indica Linn. (Family: Fabaceae). It is a neutral, nonionic, and branched polysaccharide having good water solubility, hydrophilic, gel-gel-forming, and mucoadhesive properties. TG is biodegradable, biocompatible, noncarcinogenic and nonirritant. Chemically it is highly branched carbohydrate polymer. Its backbone consists of D- glucose units joined with (1-4) b-linkages similar to that of cellulose. [7] Acyclovir anti-viral drug was used as a model drug candidate.
Materials and Methods
Materials
Acyclovir was obtained from Julphar Gulf Pharmaceutical Industries, Ras al khaimah, UAE. Lactose monohydrate (VWR International, Germany), HPMC (HiMedia Laboratories, India), Talc (LobaChemie, India), Mg stearate (Sigma Aldrich, Germany) and Tamarind seeds were obtained from local markets of Ras Al Khaimah, UAE.
Extraction of tamarind gum
Modified method of Newton AMJ et al., was used for the extraction. The tamarind seeds were collected and washed with distilled water.
The seeds were crushed and the outer covering layer was removed completely. The seed were then crushed and boiled in glass container containing distilled water for 1 h and thick slurry was obtained. Acetone was added to the slurry and gum was precipitated. It was dried at 50⁰C in hot air-oven. The dried material was further powdered and used. [5]
Fourier-Transform Infrared (FTIR) spectroscopy: The infrared spectroscopic analysis of acyclovir, tamarind gum and formulation was performed using Agilent Model Cary 630.
Differential Scanning Calorimetry (DSC): DSC studies were performed to find out any interaction between acyclovir and tamarind gum. The calorimeter (Shimadzu DSC 60+) was run at a scanning speed of 10°C/min. The temperature range of heating was 25°C–400°C. After sealing the samples in aluminum pans, heating was carried out in an inert atmosphere which was maintained by circulating nitrogen gas.
Standard curve for the assessment of Acyclovir
Buffer (pH 6.0) was used as diluent to prepare the dilution of drug for the construction of calibration curve. Pipetted out, 1, 2, 3, 4 and 5 ml from stock solution and transfer into a 10 ml volumetric flask separately. The volume was made up with diluent and finally concentration of 2, 4, 6, 8 and 10 μg/ml were prepared, respectively. These solutions were quantified at λ=256 nm.
Experimental design
USP-36 was used to obtain the basic knowledge about the behavior and properties of other excipients and their fraction [Table 1]. A simple and more effective method of direct compression was used to compress the powder blends. [8]
| Table 1: Composition of Acyclovir bio adhesive vaginal tablets | ||||||
|---|---|---|---|---|---|---|
| Ingredients (mg) | Formulation | |||||
| FT | F1 | F2 | F3 | F4 | F5 | |
| Acyclovir | 300 | 300 | 300 | 300 | 300 | 300 |
| HPMC | 75 | 100 | 75 | 100 | 125 | 150 |
| Tamarind gum | 75 | ----- | 75 | 100 | 125 | 150 |
| Lactose | ---- | 390 | 340 | 290 | 240 | 190 |
| Talc | 340 | --- | ----- | ---- | ----- | ---- |
| Magnesium stearate | 10 | 10 | 10 | 10 | 10 | 10 |
| Total weight | 800 | 800 | 800 | 800 | 800 | 800 |
Preparation of tablets by direct compression method
The tablets were initially prepared by using talc as filler, whereas all the other ingredients of the formulation were same as that of Table 1. The appearance of tablets was not good, and the friability and hardness results were also not satisfactory.
Another set of tablets was made with lactose in place of talc, and they were assessed for various parameters. All the ingredients such as Acyclovir, HPMC, tamarind gum and lactose were accurately measured and transferred into polybag for thorough and uniform mixing of powder (approx. 5 minutes). The powder sieved with mesh # 40 for uniformity of particle size and distribution. Finally, the Magnesium stearate was included into the powder blends and compressed on a 16 Station CadMach CMD 3 rotary tableting machineby using 19.0 mm Oblong shape plain punches at a theoretical weight of 800 mg ± 5.0%.
Pharmaceutical study of formulated tablets
Total 6 batches were prepared, and these tablets were subjected to recommended pharmacopeial assessments.
Weight variation: It is supposed that if the weight of tablets are uniform, it means the content of drug will be uniform thought out the batch of tablets. The weight variation test was done by weighing 20 tablets from each batch by using electronic balance and calculated the mean and standard deviation of tablets weight. [9]
Hardness and thickness: The disintegration and friability of tablets is influenced by the potential of a tablet to resist at particular pressure before breaking. The packaging and delivery of solid dosage form is greatly affected by the thickness, diameter, and length parameters. 10 tablets from each batch were measured for hardness, thickness, and diameter by using hardness tester and vernier caliper.
Friability test: The proposed methods of USP and BP were used to measure the friability of all formulated tablets. This test helps to predict the durability of tablets during their manufacturing and packaging processes which is inspected by the percentage of broken tablet mass. [10,11]
Water uptake studies: Weighed accurately 3 Tablets (W0) and transferred in 10 mL petri dishes separately. Then tablets were immersed in 50 mL of buffer (pH=6.0). Samples were observed after 15, 30, 45, 60 and 120 min. The percentage increase in weight due to absorbed liquid or water uptake was estimated by using equation: [12]
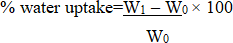
In Vitro drug release studies: Drug release studies were carried out using dissolution apparatus type II in 500 ml of acetate buffer pH 6 at 25 rpm for 12 hrs. [13]
Biodhesion study: Bioadhesion study was carried out using modified balance method. Isolated sheep vaginal tissue was fixed to steel piece with cyanoacrylate adhesive. This was kept in a beaker and then acetate buffer (pH 6), pre-warmed to 37°C, was added to the beaker to cover the upper surface of the mucosa in order to maintain its viability. Water was added dropwise the addition of water was stopped when the bioadhesive system was detached from the mucosa. The weight required to detach the system from the mucosa was noted.
Results and Discussion
Construction of calibration curve
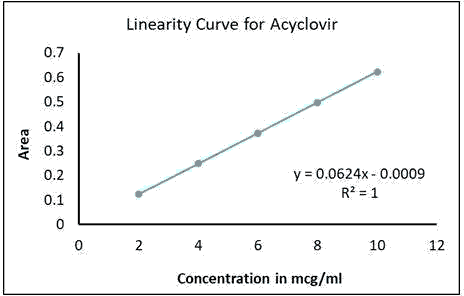
The standardization curve for acyclovir was linear in the concentration range of 2.0-10.0 μg/ml with a regression analysis (r²=1.00) as shown in Figure 1. The curve was plotted for absorbance versus concentration. The linearity between absorbance and concentration is proving that the method is acceptable for its intended purpose [Figures 1 and 2].
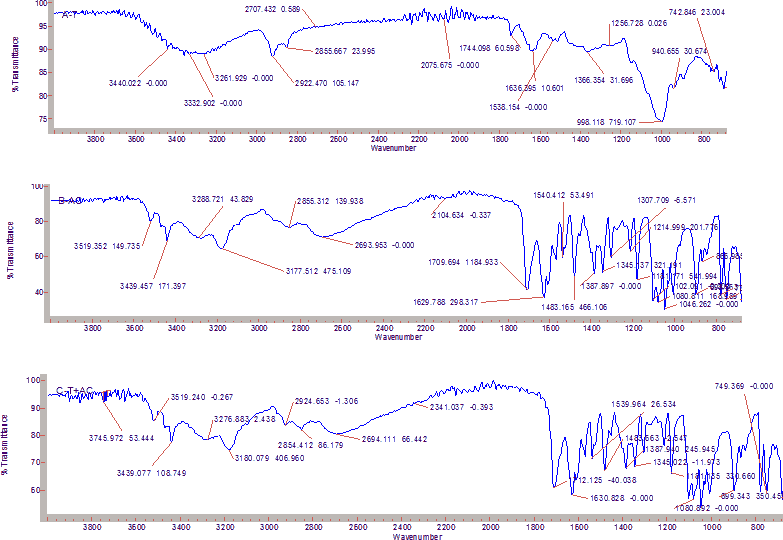
Fourier-Transform Infrared (FTIR) spectroscopy Figure 2 shows the spectra of tamarind, acyclovir and formulation containing drug and gum.
Pure drug and formulation were subjected to compatibility testing by spectroscopic analysis by FT-IR method for to determine if there is any interaction between the polymers and drug used. The tamarind gum spectra showed peaks at 1636, 2922 and 3444 which are associated with the groups C=O, C-H and OH respectively. The spectra of acyclovir exhibited peaks at 3519(OH), 1629(C=O), 3288(NH2) and at 1483(C=N). The major peaks were not altered in the formulation containing tamarind and acyclovir which shows that there is no interaction between the gum used and the drug. It can be said that they are compatible with each other in the formulation [Figures 3-5].
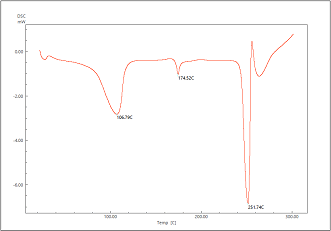
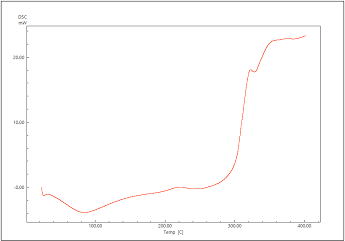
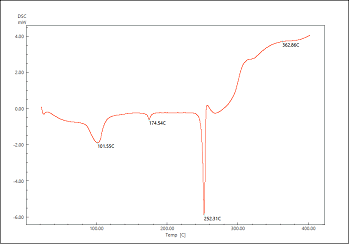
Differential Scanning Calorimetry (DSC)
The DSC spectra of acyclovir, tamarind and formulation are shown in Figure 3-5. Acyclovir showed a sharp peak at 251⁰ C which is dying to melting of the drug. The sharp peak remained same in the formulation along with tamarind gum suggesting that there is no chemical interaction between drug and gum.
Quality assessment of formulated tablets
The tablets were analyzed for various physical parameters. The physical features of tablets like weight variation, thickness, hardness, friability, and drug dissolution must be controlled in production of tablets to assure not only the external appearance of the tablets but also its therapeutic efficacy.
The physical assessment of all formulated tablets was done. The appearances were elegant, capsulated in shape and white in color. All tablets were found to be within the acceptable limit according to the USP (± 5%) 4. Average weight of tablets made by talc was 793.77 ± 2.42 whereas the lactose fell in the range from (Mean ± % CV) 795.36 ± 1.72 to 817.38 ± 1.56 mg, indicating the less deviations among the weight of individual tablets as compared to that of talc tablets [Table 2]. Control of weight variation theoretically assure the content uniformity of drug substance in tablets.
| Table 2: Indicating the less deviations among the weight of individual tablets | |||||
|---|---|---|---|---|---|
| Formulations | Weight variation (Mean ± %CV) | Friability (% w/w) | Hardness (kp) (Mean ± SD) | Length (mm) (Mean ± SD) | Thickness (mm) (Mean ± SD) |
| FT | 793.77 ± 2.42 | 4.36 | 4.5 ± 1.08 | 20.25 ± 0.173 | 4.09 ± 0.063 |
| F1 | 807.99 ± 2.62 | 0.772 | 11.67 ± 1.43 | 20.23 ± 0.096 | 4.56 ± 0.104 |
| F2 | 795.36 ± 1.72 | 1.72 | 7.83 ± 0.47 | 20.14 ± 0.048 | 4.68 ± 0.029 |
| F3 | 806.62 ± 1.92 | 0.963 | 9.0 ± 1.08 | 20.25 ± 0.041 | 4.68 ± 0.029 |
| F4 | 807.95 ± 2.04 | 1.931 | 7.83 ± 0.24 | 20.23 ± 0.05 | 4.7 ± 0.082 |
| F5 | 817.38 ± 1.56 | 1.736 | 7.67 ± 0.24 | 20.21 ± 0.085 | 4.88 ± 0.05 |
Ten (10) tablets from each formulation were selected randomly and measured individually for thickness and length using verniercaliper [Table 2]. The thickness was found in the range from 4.56 to 4.88 mm with ± SD between 0.029-0.104, length from 20.14 ± 0.048 to 20.25 ± 0.041 mm for lactose and 20.25 ± 0.173 mm for talc tablets that indicating more variation in length. For proper packaging, the accuracy in shape of tablets has great impact. It helps to control the dispensing of counterfeit drugs in the market and regulate functionality of drug products.
Ten (10) tablets were selected and measured for hardness in Kilopond (kp) [Table 2]. For the handling and shipping of tablets, must have good mechanical strength. The main portion of tablet consists of filler, so the selection of appropriate and compatible filler is important in the construction of tablets characteristics. Hardness of tablets prepared by using talc was found to be 4.5 ± 1.08 kp, near to lower limit of standard value (4–6 kp) whereas the F1-F5 tablets ranged from 7.67 ± 0.24 to 11.67 ± 1.43 Kp [Table 2]. As per United States Pharmacopeia, friability of 10 tablets from each formulation was done by using Veego Friability Apparatus [Table 2]. Friability values for FT was found 4.36% whereas for F1-F5 in the range from 0.772%-1.72% (i.e. <1%) [Table 2]. In case of Ft, the tablets damaged after tumbling, indicating fragility of the preparation.
Conclusion
The vaginal tablets prepared using tamarind gum exhibited good physical characteristics and showed sustained drug release. Bioadhesion studies showed that the tablets are having good bioadhesion properties. The prepared tablets can be effectively used as a vaginal dosage form.
Acknowledgements
The authors express their gratitude to the President of RAK Medical and Health Sciences University, UAE and Dean RAK College of Pharmaceutical Sciences for their encouragement and support in carrying out the research. The authors also thank Julphar, Gulf Pharmaceutical Industries, Ras Al Khaimah, UAE to provide gift sample of Acylovir.
REFERENCES
- Notario-Perez F, Ruiz-Caro R, Veiga-Ochoa MD. Historical development of vaginal microbicides to prevent sexual transmission of HIV in women: From past failures to future hopes. Drug Des Devel Ther. 2017;11:1767-1787.
- Kast CE, Valenta C, Leopold M, Bernkop-Schnürch , A. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clotrimazole. J Contr Rel. 2002; 81: 347-354.
- Christopher M, Ian M, Brid D, Andrew B. Development of a multi-layered vaginal tablet containing dapivirine, levonorgestrel and acyclovir for use as a multipurpose prevention technology. Euro J of Pharm and biopharm. 2016; 104:171-179.
- Newtona AM, Indanab VL, Jatinder K. Chronotherapeutic drug delivery of tamarind gum, chitosan and okragum controlled release colon targeted directly compressedpropranolol HCL matrix tablets and in-vitro evaluation. Int J of Biol Macro. 2015; 79: 290-299.
- Vasantrao PS, Dhawale SC. Tamarind gum: A pharmaceutical overview. Pharmaceutical reviews. 2008; 6(4): 1-6.
- Nayak A, Pal D. Tamarind seed polysaccharide: An emerging excipient for pharmaceutical use. Indian J Pharm Educ Res. 2017; 51(2):136-146.
- United States Pharmacopeial. Revision Bulletin. United States Pharmacopeial Convention 2010.
- United States Pharmacopeia and National Formulary (USP 36-NF 31). Uniformity of Dosage Units, Weight Variation <905>, Rockville, MD: United States Pharmacopeia Convention. 2013; p: 431-434.
- Varatharajan P, Sabarikumar K, AntoShering M, lavarasan P. Formulation and evaluation of voglibose mouth dissolving tablets by direct compression method. Journal of Pharmacy Research. 2012; 5(2): 749-754.
- Sharma J, SrinivasaRao A. Formulation optimization and evaluation of tramadol oral disintegrating tablets. International Journal of Pharmaceutical Sciences Review & Research. 2013; 18(2): 38-41.
- Gurumurthy V, Deveswaran R, Bharath S, Basavaraj BV, Madhavan V. Design and optimization of bioadhesive vaginal tablets of acyclovir. Ind J Pharm Edu Res. 2013; 47(2): 140-147.
- Sudeendra RB, Shivakumar HG. Bioadhesive controlled release clotrimazole vaginal tablets. Tropical J Pharm Res. 2010; 9: 339-346.
- Rao YM. Design and evaluation of mucoadesive drug delivery system. Indian Drugs. 1998; 35: 558-565.
Select your language of interest to view the total content in your interested language
Awards Nomination
20+ Million Readerbase
Google Scholar citation report
Citations : 24805
Annals of Medical and Health Sciences Research received 24805 citations as per google scholar report
Annals of Medical and Health Sciences Research peer review process verified at publons
Indexed in
PubMed Central Index Copernicus Emerging Sources Citation Index
Abstracted/Indexed in
- Include Baidu Scholar
- CNKI (China National Knowledge Infrastructure)
- EBSCO Publishing's Electronic Databases
- Exlibris – Primo Central
- Google Scholar
- Hinari
- Infotrieve
- National Science Library
- ProQuest
- TdNet
- African Index Medicus
 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.