Gestational Diabetes Mellitus and its Correlation with Family History of Diabetes Mellitus: Systematic Review and Meta-analysis
2 Medical Intern, King Faisal University, Alahsa, Saudi Arabia
3 General Practitioner, Albukayriah General Hospital, Qassim-Albukayriah, Saudi Arabia
4 Medical Intern, Taibah University, Madina, Saudi Arabia
5 General Practitioner, Alaqiq First Primary Health Care Center Albaha, Aqiq-Albaha, Saudi Arabia
6 Medical Intern, Almaarefa University, Riyadh, Saudi Arabia
7 General Practitioner, King Abdullah Medical Complex, Jeddah, Saudi Arabia
8 General Practitioner, Imam Muhammad Ibn Saud Islamic University,Riyadh, Saudi Arabia
9 General Practitioner, Taibah University, Madinah, Saudi Arabia
10 Medical Intern, Taif University, Taif, Saudi Arabia
11 Resident, Ibn Sina College, Jeddah, Saudi Arabia
12 Home Care Resident, Asser Center Hospital MOH, Abha, Saudi Arabia
13 General Practitioner, Alkhobar Primary Health Care Sector, E1 Cluster, Ministry of Health, Alkhobar, Saudi Arabia
14 General Practitioner, Dammam, Saudi Arabia
15 General Practitioner, King Abdulaziz University, Jeddah, Saudi Arabia
Published: 31-Dec-2021
Citation: Alazmi AAF, et al. Gestational Diabetes Mellitus and its Correlation with Family History of Diabetes Mellitus: Systematic Review and Meta-analysis. Ann Med Health Sci Res. 2021;11:26-30.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Gestational Diabetes Mellitus (GDM), a common obstetric complication, is defined as any degree of glucose intolerance with onset or first popularity at some point of being pregnant, because traditional hazard factors, which include maternal age, weight problems, and ethnicity, do now not explain the superiority of GDM within the pregnant population, there’s good-sized interest in spotting nontraditional, novel chance factors e.g. family history of DM. Aim: This work aims to determine Gestational Diabetes Mellitus (GDM) and its correlation with the family history of Diabetes Mellitus (DM) patients. Literature Review: A systematic search was performed over different medical databases to identify family medicine studies, which studied the outcome of the GDM group versus the Non-GDM group of pregnant patients. Using the meta-analysis process, either with fixed or random-effects models, we conducted a meta-analysis on the family history of DM as a primary outcome, and on the previous maternal history of GDM as a secondary outcome. Nine studies were identified involving 7432 patients, with 997 patients in the GDM group, and 6435 patients in the Non-GDM group. The meta-analysis process revealed that the random-effects model of the meta-analysis process revealed a highly significant increase in the family history of DM and previous maternal history of GDM in the GDM group compared to the Non-GDM group (p<0.01 respectively). Conclusion: To conclude, Gestational Diabetes Mellitus (GDM), a common obstetric complication, is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. Because traditional risk factors, such as maternal age, obesity, and ethnicity do not completely explain the prevalence of GDM in the pregnant population, there is considerable interest in recognizing nontraditional, novel risk factors. Family history of DM has an important role in gestational diabetes.
Keywords
Gestational diabetes mellitus; Family history; Diabetes mellitus
Introduction
Gestational Diabetes Mellitus (GDM) is a common problem of being pregnant. It’s far related to an expansion of maternal and infant perinatal headaches, which include preeclampsia, gestational high blood pressure, hydramnios in moms, and preterm beginning, hypoglycemia, macrosomia in infants, and so on. The pathogenesis of GDM continues to be now not completely understood, however, it is very similar to that of Type 2 Diabetes Mellitus (T2DM), in which strange pancreatic insulin release and Insulin Resistance (IR) being involved.[1]
Gestational Diabetes Mellitus (GDM) has an incidence starting from 2% to 22% international depending on the population and sort of diagnostic check and the ladies with GDM are liable to Impaired Glucose Tolerance (IGT) and Diabetes Mellitus (DM) later in lifestyles. The use of early postpartum screening to rule out appears diabetes located at some stage in pregnancy and located that any diploma of glucose intolerance after being pregnant became as strong a predictor for future diabetes as GDM itself.[2]
Gestational Diabetes Mellitus (GDM), a common obstetric complication, is defined as any degree of glucose intolerance with onset or first popularity at some point of being pregnant. Because traditional hazard factors, which include maternal age, weight problems, and ethnicity, do now not explain the superiority of GDM within the pregnant population, there's good-sized interest in spotting non traditional, novel chance factors e.g. family history of DM. The latest research has centered on the risk assessment of exposure to metals from each nature and environmental pollutants, which can also have a power on the development of unfavorable pregnancy consequences, along with GDM. In day-by-day lifestyles, more than one metals have ubiquitous get admission to the public thru ambient air, drinking water, food, medications and consumer products.[3]
The timely detection of glucose tolerance postpartum is important because progression to DM2 can be decreased by about forty percent by using implementing a way of life intervention programs after being pregnant. To obtain this aim, organizations including the yank university of obstetricians and gynecologists (ACOG), the American Diabetes Affiliation (ADA), the worldwide Federation of Gynecology and Obstetrics (FIGO), the National Institute for fitness and care excellence (great) suggest that all ladies who increase GDM undergo glucose tolerance checking out at 6 weeks–12 weeks postpartum, especially through an Oral Glucose Tolerance Test (OGTT).[4]
This work aims to determine Gestational Diabetes Mellitus (GDM) and its correlation with the family history of Diabetes Mellitus (DM) patients.
Literature Review
Our review came following the (PRISMA) statement guidelines.[5]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing pregnant patients.
The excluded studies were non-english, or animal studies or describing non-pregnant patients.
Study identification
Basic searching was done over the PubMed, Cochrane library, and Google scholar using the following keywords: Gestational diabetes mellitus, family history, diabetes mellitus.
Data extraction
Comparative studies, clinical trials, and Randomized Controlled Trials (RCTs), which studied the outcome of the GDM group vs. non-GDM group of pregnant patients, will be reviewed.
Outcome measures included family history of DM as a primary outcome and previous maternal history of GDM as a secondary outcome.
Study selection
We found 250 records, 190 excluded because of the title; 60 articles are searched for eligibility by full-text review; 22 articles cannot be accessed; 13 studies were reviews and case reports; 16 were not describing the functional outcome.
The studies which met all inclusion criteria were 9 studies.
Statistical Analysis
Pooled Odds Ratios (OR), Proportions (%), with 95% Confidence Intervals (CI) assessed, using a statistical package (MedCalc, Belgium). The meta-analysis process was established via I2-statistics (either the fixed-effects model or the random-effects model), according to the Q test for heterogeneity.
The included studies were published between 2010 and 2021. Regarding the type of included studies, 3 studies (out of 9 studies) were prospective, 4 were cross-sectional, while 2 studies were retrospective [Table 1].
Regarding patients’ characteristics, the total number of patients in all the included studies was 7432 patients, with 997 patients in the GDM group, and 6435 patients in the non-GDM group [Table 1].
The mean maternal age of all patients was (29.16 years), and the mean BMI was (25.6) [Table 1].
| N | Author | Type of study | Number of patients | BMI (average) | Maternal age (average years) | ||
|---|---|---|---|---|---|---|---|
| Total | GDM group | Non-GDM group | |||||
| 1 | Soheylikhah et al., [6] | Prospective | 605 | 97 | 508 | 28.5 | 27 |
| 2 | Khan et al., [7] | Retrospective | 200 | 103 | 97 | 28 | 33 |
| 3 | Erem et al., [8] | Prospective | 815 | 39 | 776 | 26 | 30 |
| 4 | Lin et al., [9] | Retrospective | 512 | 106 | 406 | 22 | 31 |
| 5 | Begum et al., [10] | Cross-sectional | 303 | 22 | 281 | 20 | 23 |
| 6 | Macaulay et al., [11] | Cross-sectional | 1900 | 174 | 1726 | 29.5 | 30 |
| 7 | Groof et al., [12] | Cross-sectional | 973 | 108 | 865 | --- | 30 |
| 8 | Chanda et al., [13] | Cross-sectional | 1212 | 202 | 1010 | --- | 23.5 |
| 9 | Lewandowska et al., [14] | Prospective | 912 | 146 | 766 | --- | 35 |
Table 1: Patients and study characteristics.
Our meta-analysis included 9 studies comparing 2 different groups of patients; with a total number of patients (N=7432) [Table 2].
| N | Author | Primary outcome | Secondary outcome | ||
|---|---|---|---|---|---|
| Family history of DM | Previous maternal history of GDM | ||||
| GDM group | Non-GDM group | GDM group | Non-GDM group | ||
| 1 | Soheylikhah et al. | 74 | 230 | 7 | 6 |
| 2 | Khan et al. | 87 | 26 | 78 | 0 |
| 3 | Erem et al. | 27 | 215 | 2 | 2 |
| 4 | Lin et al. | 66 | 76 | --- | --- |
| 5 | Begum et al. | 4 | 45 | 1 | 3 |
| 6 | Macaulay et al. | 56 | 419 | --- | --- |
| 7 | Groof et al. | 76 | 503 | 50 | 363 |
| 8 | Chanda et al. | 24 | 51 | --- | --- |
| 9 | Lewandowska et al. | 90 | 265 | 8 | 3 |
Table 2: Summary of outcome measures in all studies.
Each outcome was measured by
•Odds Ratio (OR)
•For the family history of DM.
•For the previous maternal history of GDM.
Concerning the primary outcome measure,
We found 9 studies reported a family history of DM.
I2 (inconsistency) was 87.6%, Q test for heterogeneity (p< 0.001), so random-effects model was carried out; with overall OR=3.39 (95% CI=2.106 to 5.459).
The random-effects model of the meta-analysis process revealed a highly significant increase in the family history of DM in the GDM group compared to the non-GDM group (p<0.01) [Figure 1].
Concerning the secondary outcome measures,
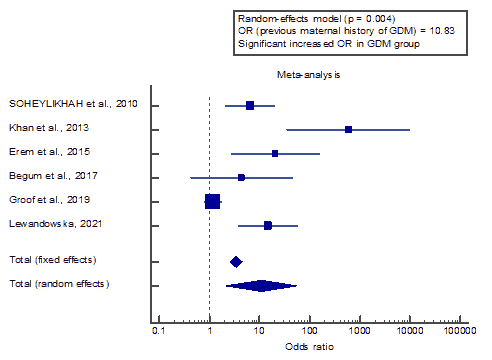
We found 6 studies that reported previous maternal history of GDM.
I2 (inconsistency) was 89.6%, Q test for heterogeneity (p<0.001), so random-effects model was carried out; with overall OR=10.83 (95% CI=2.096 to 55.994).
The random-effects model of the meta-analysis process revealed a highly significant increase in the previous maternal history of GDM in the GDM group compared to the non-GDM group (p=0.004).
Discussion
This work aims to determine Gestational Diabetes Mellitus (GDM) and its correlation with the family history of Diabetes Mellitus (DM) patients.
The included studies were published between 2010 and 2021. Regarding the type of included studies, 3 studies (out of 9 studies) were prospective, 4 were cross-sectional, while 2 studies were retrospective.
Regarding patients’ characteristics, the total number of patients in all the included studies was 7432 patients, with 997 patients in the GDM group, and 6435 patients in the non-GDM group.
The mean maternal age of all patients was (29.16 years), and the mean BMI was (25.6). Our meta-analysis included 9 studies comparing 2 different groups of patients; with a total number of patients (N=7432).
Concerning the primary outcome measure, we found 9 studies that reported a family history of DM.
The random-effects model of the meta-analysis process revealed a highly significant increase in the family history of DM in the GDM group compared to the non-GDM group (p<0.01). Which came in agreement with Hoorn et al.,[3] Aagaard et al.,[2] Chanda et al.,[15] Lewandowska et al.,[16] Lee et al.,[17] Li et al.,[18] Bogdanet et al.,[19] Deng et al.[1] and Dalfrà et al.[4]
Van Hoorn et al., reported that in step with Dutch scientific pointers, girls have been considered at high risk for GDM if they had one or more pre specified danger elements: BMI >30 kg/m2, previous child with a birth weight above the 95th centile or 4500 g, history of GDM, a first-degree family member with any sort of diabetes mellitus, non-western ethnicity with a high prevalence of diabetes mellitus.[3]
Aagaard et al. reported that they determined a 70% diabetes rate 25 years after pregnancy. The most effective family history of diabetes in cohort 2 was associated with conversion to occur diabetes (p<0.01), additionally after adjustment for age, BMI, non-danish origin, and smoking during pregnancy (p<0.01).[2]
Chanda et al. reported that ladies who passed tenth elegance (aOR=1.58), belonging to the muslim religion (OR=1.52) and above the poverty line (APL; OR=1.38) had a significantly extended chance of developing GDM compared with respective baseline groups (p<0.05). Gravida status, BMI, and being non-anemic were non-significant chance factors for GDM. Family history of diabetes (OR=1.82) and smoking (OR=1.61) have been significant and independent predictors of GDM, found GDM prevalence of 16.7% in rural Assam (range: 12.9%- 20.6%). Training (tenth bypass), age (26 years-30 years), religion (muslim), socioeconomic status (APL), tobacco use (presently smokers), and beyond records (own family history of diabetes) have been the massive primary predictors of GDM in rural Assam.[15]
Lewandowska et al. reported that a few categories of the family history had been impartial chance elements of GDM-1 or GDM-2. Compared to ‘absence of diabetes within the family’, girls with diabetes in the father had a 3.60 eight-fold growth in GDM-1 hazard (AOR-b=3.68 (2.23-6.07)), and ladies with diabetes in the mother had a 2.13-fold increase in GDM-1 chance (AOR-b=2.13(1.1-4.14)) and a 4.73- fold growth in GDM-2 threat (AOR-b=4.73 (1.26-17.77)). Girls with diabetes inside the grandmother had a 2.34-fold increase in GDM-1 hazard (AOR-b=2.34 (1.29-4.24)).[16]
Lee et al. reported that more than one-half of the respondents had a family history of diabetes (56.5%) and high blood pressure (52.9%), whilst more than one-third had a family record of GDM (34.8%). Fine of lifestyles is an indicator of properly-being within the pregnant mom. But, almost one-quarter of women with GDM have negative-to-moderate QOL. Moreover, women with GDM who have their family records of depression, tension, and a family history of GDM, in addition to folks that received insulin without or with OHA, and/or are on diet change, have better odds of getting poor-to-slight QOL.[17]
Li et al. reported that family history of diabetes in first-degree relatives, gestational age at OGTT, and fasting and 2 h PG during OGTT in the course of pregnancy had been similar between the intensive care group and the standard care group. All these baseline variables were also comparable by using intervention amongst women who had been lost to follow-up.[18]
Bogdanet et al. reported that multivariate analysis using more than one logistic regression changed into done to model relationships among weight gain less than that advocated with the aid of the IOM and maternal and toddler outcomes, correcting for age, smoking status, ethnicity, and family records of diabetes (first-degree relatives).[19]
Deng et al. reported that, in a meta-analysis, the overall OR of family history of T2DM for developing GDM was estimated as 3.46, which was similar to the result in our study (OR=3.09).[1]
Dalfrà et al. reported that a positive family history of DM2 and a previous pregnancy with GDM were also significantly more common in the AGT group than in the NGT group.[4]
Concerning the secondary outcome measures, we found 6 studies that reported previous maternal history of GDM. The random-effects model of the meta-analysis process revealed a highly significant increase in the previous maternal history of GDM in the GDM group compared to the non-GDM group (p=0.004). Which came in agreement with Venkataramani et al.,[19] Aagaard et al.,[2] Li et al.[18] and Dalfrà et al.[4]
Venkataramani et al. reported that women with a history of Gestational Diabetes Mellitus (GDM) are at increased hazard for type 2 diabetes and for that reason require ordinary follow-up screening for diabetes; however, many women do no longer receive this screening, and especially low-income girls face disparities in receipt of recommended observe-up care. At the same time as those ladies may also have limited get admission to healthcare following pregnancy.
Aagaard et al., reported that, 15 years after pregnancy and after 40 years of age. The women of cohort 2 with recent GDM are at risk of DM at a higher charge. on this background, our outcomes are beneficial in identifying the time in which GDM girls can also enjoy the effective implementation of evidence-based remedies to postpone and ad appear DM.[2]
Li et al. reported that growing evidence suggests that girls with a history of GDM have a markedly improved chance of diabetes later in life, but it's far still unknown whether in-depth control of GDM has benefits for the prevention of maternal diabetes at postpartum and later in life.[18]
Dalfrà et al. reported that, belonging to an immigrant ethnic group, having a positive family record of type 2 diabetes, and wanting insulin therapy for the duration of pregnancy emerged as the best predictors of AGT developing after being pregnant complicated using GDM. These findings are steady with those of Lawrence.[4]
Conclusion
To conclude Gestational Diabetes Mellitus (GDM), a common obstetric complication, is defined as any degree of glucose intolerance with onset or first recognition during pregnancy. Because traditional risk factors, such as maternal age, obesity, and ethnicity do not completely explain the prevalence of GDM in the pregnant population, there is considerable interest in recognizing nontraditional, novel risk factors. Family history of DM has an important role in gestational diabetes.
Authorship
All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
References
- Deng M, Zhou J, Tang Z, Xiang J, Yi J, Peng Y, et al. The correlation between plasma total homocysteine level and gestational diabetes mellitus in a Chinese han population. Sci Rep. 2020;10:1-5.
- Aagaard KA, Al-Far HM, Piscator U, Krogh RA, Lauszus FF. Manifest diabetes after gestational diabetes: A double-cohort, long-term follow-up in a Danish population. Arch Gynecol. Obstet. 2020;302:1271-8.
- van Hoorn F, Koster MP, Naaktgeboren CA, Groenendaal F, Kwee A, Ruiter M, et al. Prognostic models single risk factor approach in first-trimester selective screening for gestational diabetes mellitus: A prospective population-based multicentre cohort study. Int J Gynecol Obstet. 2021;128:645-54.
- Dalfrà MG, Burlina S, Del Vescovo GG, Anti F, Lapolla A. Adherence to a follow-up program after gestational diabetes. Acta Diabetologica. 2020;57:1473-80.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. BMJ. 2009;339.
- Khan R, Ali K, Khan Z. Socio-demographic risk factors of gestational diabetes mellitus. Pak J Med Sci. 2013;29:843.
- Erem C, Kuzu UB, Deger O, Can G. Prevalence of gestational diabetes mellitus and associated risk factors in Turkish women: The trabzon GDM Study. Arch Med Sci. 2015;11:724.
- Lin PC, Hung CH, Chan TF, Lin KC, Hsu YY, Tzeng YL. The risk factors for gestational diabetes mellitus: A retrospective study. Midwifery. 2016;42:16-20.
- Begum P, Shaha DR, Walid KM. Gestational diabetes mellitus and associated risk factors in patients attending diabetic association medical college hospital in Faridpur. J Enam Med Coll. 2017;7:126-33.
- Macaulay S, Ngobeni M, Dunger DB, Norris SA. The prevalence of gestational diabetes mellitus amongst black South African women is a public health concern. Diabetes Res Clin Pract. 2018;139:278-87.
- Groof Z, Garashi G, Husain H, Owayed S, AlBader S, Mouhsen H, et al. Prevalence, risk factors, and fetomaternal outcomes of gestational diabetes mellitus in Kuwait: A cross-sectional study. J Diabetes Res. 2019;2019.
- Chanda S, Dogra V, Hazarika N, Bambrah H, Sudke AK, Vig A, et al. Prevalence and predictors of gestational diabetes mellitus in rural Assam: A cross-sectional study using mobile medical units. BMJ Open. 2020;10:e037836.
- Lewandowska M. Gestational Diabetes Mellitus (GDM) risk for declared family history of diabetes, in combination with BMI categories. Int J Environ Res Public Health. 2021;18:6936.
- Chanda S, Dogra V, Hazarika N, Bambrah H, Sudke AK, Vig A, et al. Prevalence and predictors of gestational diabetes mellitus in rural Assam: A cross-sectional study using mobile medical units. BMJ Open. 2020;10:e037836.
- Lewandowska M. Gestational Diabetes Mellitus (GDM) risk for declared family history of diabetes, in combination with BMI categories. Int J Environ Res Public Health. 2021;18:6936.
- Lee KW, Ching SM, Hoo FK, Ramachandran V, Chong SC, Tusimin M, et al. Factors associated with poor-to-moderate quality of life among pregnant women with gestational diabetes mellitus: A cross-sectional study in Malaysia. Qual Life Res. 2020;29:2725-36.
- Li N, Liu J, Zhang C, Liu G, Leng J, Wang L, et al. Effects of lifestyle intervention during pregnancy on postpartum diabetes among Chinese women with gestational diabetes. 2021;64:255-8.
- Bogdanet D, Mustafa M, Khattak A, O’Shea PM, Dunne FP. Atlantic DIP: Is weight gain less than that recommended by IOM safe in obese women with gestational diabetes mellitus?. Int J Obes. 2021;45:1044-51.
- Venkataramani M, Cheng TL, Yeh HC, Bennett WL, Maruthur NM. Family-oriented social service touch points as opportunities to enhance diabetes screening following a history of gestational diabetes. J Am Board Fam Med. 2020;33:616-9.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.