Giant Cell Rich Malignancies: A Meta-analysis
Citation: Gore MR. Giant Cell Rich Malignancies: A Metaanalysis. Ann Med Health Sci Res. 2018;8:225-232
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: The presence of osteoclast-like giant cells in malignant tumors is a rare finding. Giant cell rich histology can be seen in both benign and malignant tumors and can be found in diverse sites from the skeleton to the head and neck to the viscera. The rarity of these tumors makes their identification and treatment a dilemma. Materials and methods: A meta-analysis of 43 case reports and case series was conducted from studies from 1989-2018, totaling 82 patients. Data on demographics, survival, stage, site, and histopathology was compiled and analyzed using Kaplan-Meier analysis/log-rank and logistical regression. Results: Giant cell rich osteosarcoma was the most common histopathological type, and limb was the most common site. On univariate analysis histopathological type, site, treatment type, and AJCC, T, N, and M stage were found to significantly affect survival, while on multivariate analysis only histopathological type was found to be significant. Conclusions: Giant cell rich malignancies are rare and can affect diverse sites. Lower stage, treatment type, histopathological type, and primary site significantly affect survival in these uncommon cancers.
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Haberler
Keywords
Giant cell rich; Cancer; Malignancy; Sarcoma; Meta-analysis
Introduction
The presence of giant cells in certain benign and malignant neoplasms has been known for some time. Flanagan and coworkers [1] noted that the multinucleated giant cells seen in giant cell granuloma were osteoclasts by noting that the giant cells excavated bone, their motility was inhibited by calcitonin and that the cells bound osteoclast-specific monoclonal antibodies. Flanagan and Chambers [2] also noted that a giant cell rich variant of malignant fibrous histiocytoma (MFH) contained osteoclast-like giant cells that excavated bone and bound osteoclast-specific monoclonal antibodies. Since that time numerous giant cell-rich lesions have been characterized, ranging from benign aneurysmal bone cysts and giant cell tumors, to malignant osteosarcomas. [3-48] These lesions are relatively rare yet can be found in diverse primary sites such as the axial skeleton head and neck, abdominal cavity and mediastinum/ thorax. Given the rarity of these giant cell malignancies and the diverse histopathological types and primary sites, treatment regimen recommendations and survival data can be difficult to determine. To our knowledge, no large meta-analysis or systematic review has examined the aggregate survival data for malignant giant cell rich tumors. In this study a literature search of case reports and case series on malignant giant cell rich tumors was conducted, and a meta-analysis was performed to examine demographic, treatment, and tumor characteristics impacting survival.
Materials and Methods
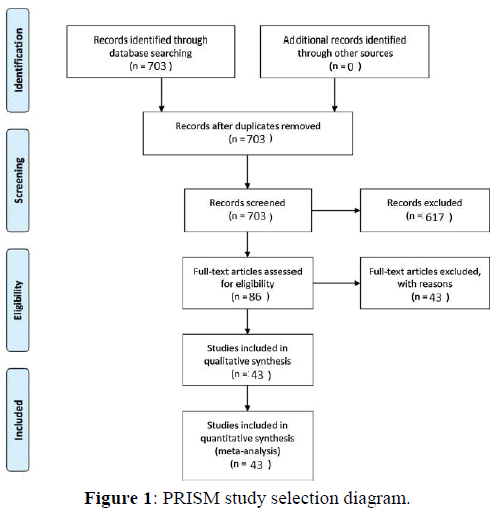
A Pubmed literature search was conducted using the search terms “giant + cell + rich”. This literature review and meta-analysis was carried out and reported using the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for the reporting of observational studies. [49] Figure 1 illustrates the PRISMA flow diagram for study selection. For the period 1989-2018 703 total papers were identified. After excluding duplicate studies, review articles, and studies without analyzable individual patient data, a total of 43 studies reporting 82 total patients were identified [5-48]. Case reports, case series, and cohort studies containing individual analyzable patient data on patients of any age with a pathologic diagnosis of a malignant giant cell rich tumor of any site were included in the meta-analysis of survival outcomes. The outcomes were overall survival and disease-free survival.
Results
A total of 82 patients with malignant giant cell rich tumors were identified from the literature. Patient demographics are summarized in Table 1. The average age was 42.7 years, the median age was 43 years, and the standard deviation for age was 22.5 years. Patient sex was unknown in 6 patients, while the remaining patients included 38 males and 38 females (1:1 M:F ratio). The most common primary sites were limb (42/82, 51.2%) and genitourinary (18/82, 22.0%). The most common AJCC (American Joint Commission on Cancer) stages were stage II and stage IV, both with 28/82 patients, 34.1% each. The most common T stage was T2 with 42/82 patients (51.2%). There were 11 of 82 patients with positive nodal metastases at diagnosis (N+, 13.4%), and 27 of 82 patients had distant metastases at diagnosis (M+, 32.9%). Table 2 summarizes the histopathological type data for the cohort. The most common histopathological subtypes were Giant cell rich (GCR) osteosarcoma (36/82, 43.9%), GCR Malignant fibrous histiocytoma (MFH, 11/82, 13.4%), and GCR endometrioid adenocarcinoma (10/82, 12.2%).
| Demographics | n | % |
|---|---|---|
| Average age=42.7 years | NA | NA |
| Median age=43 years | NA | NA |
| Age standard deviation=22.5 years | NA | NA |
| Total patients | 82 | 100% |
| Sex | ||
| F | 38/82 | 46.3% |
| M | 38/82 | 46.3% |
| Unknown sex | 6/82 | 7.3% |
| Primary site | ||
| gastrointestinal/intra-abdominal | 1/82 | 1.2% |
| genitourinary | 18/82 | 22.0% |
| head and neck | 8/82 | 9.8% |
| limb | 42/82 | 51.2% |
| organ/soft tissue NOS | 8/82 | 9.8% |
| AJCC stage | ||
| I | 4/82 | 4.9% |
| II | 28/82 | 34.1% |
| III | 8/82 | 9.8% |
| IV | 28/82 | 34.1% |
| Unknown stage | 14/82 | 17.1% |
| T (tumor) stage | ||
| T1 | 6/82 | 7.3% |
| T2 | 42/82 | 51.2% |
| T3 | 7/82 | 8.5% |
| T4 | 2/82 | 2.4% |
| TX | 25/82 | 30.5% |
| N (nodal) stage | ||
| N+ | 11/82 | 13.4% |
| N0 | 38/82 | 46.3% |
| NX | 33/82 | 46.3% |
| M (distant metastasis) stage | ||
| M+ | 27/82 | 32.9% |
| M0 | 34/82 | 41.5% |
| MX | 21/82 | 25.6% |
Table 1: Patient demographics.
| Histopathological type | n | % |
|---|---|---|
| GCR ESOS | 7/82 | 8.5% |
| GCR Endometrioid adenocarcinoma | 10/82 | 12.2% |
| GCR Leiomyosarcoma | 1/82 | 1.2% |
| GCR angiosarcoma | 1/82 | 1.2% |
| GCR atypical fibroxanthoma | 1/82 | 1.2% |
| GCR chondrosarcoma | 1/82 | 1.2% |
| GCR gliosarcoma | 1/82 | 1.2% |
| GCR hemangiopericytoma/giant cell angiofibroma | 1/82 | 1.2% |
| GCR malignant fibrous histiocytoma | 11/82 | 13.4% |
| GCR melanoma | 2/82 | 2.4% |
| GCR metaplastic carcinoma | 1/82 | 1.2% |
| GCR osteosarcoma | 36/82 | 43.9% |
| GCR squamous cell carcinoma | 1/82 | 1.2% |
| GCR undifferentiated carcinoma of the urinary tract | 6/82 | 7.3% |
| Pleomorphic giant cell-rich hepatocellular carcinoma | 1/82 | 1.2% |
| Malignant giant cell tumor of bone | 1/82 | 1.2% |
Table 2: Histopathology distribution for the cohort.
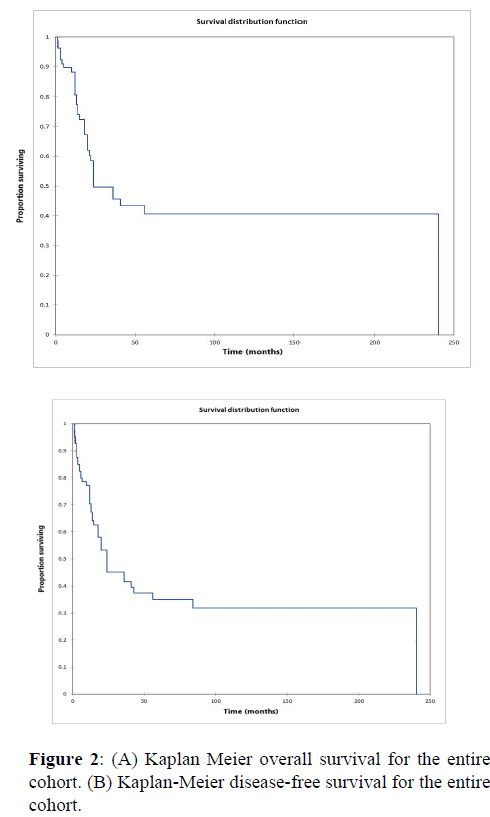
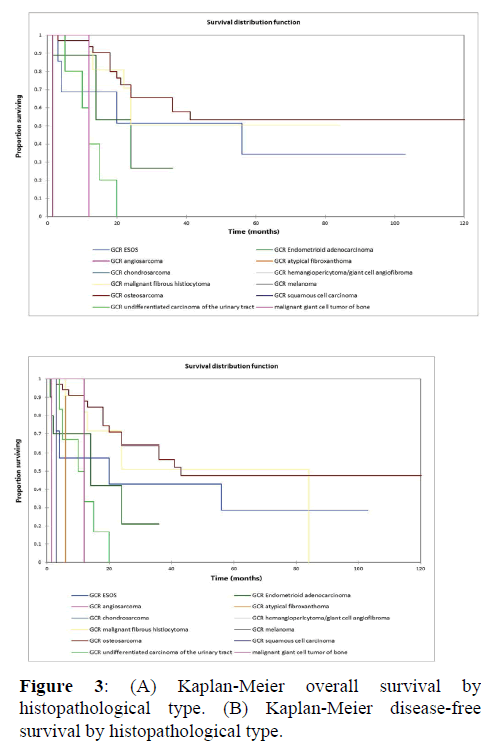
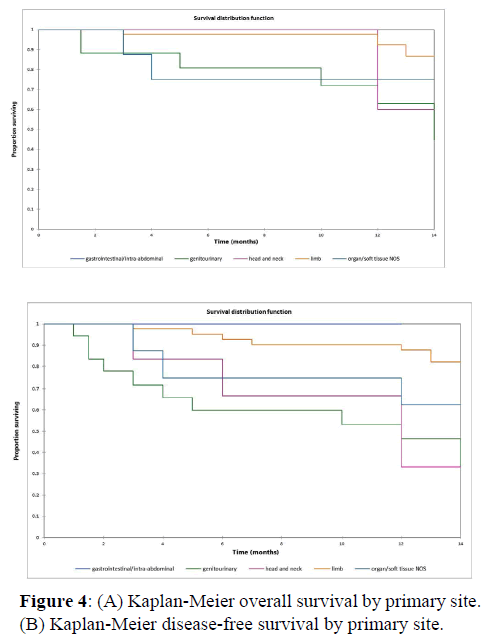
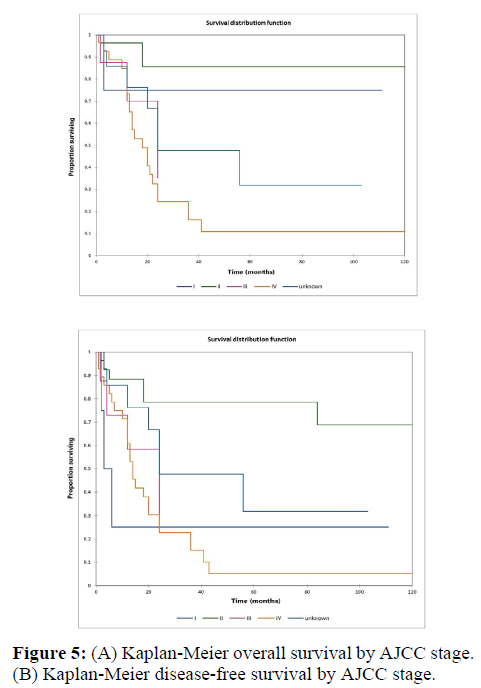
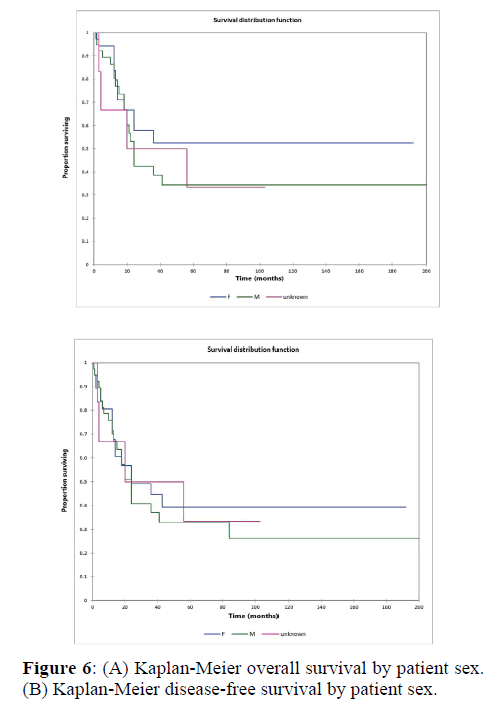
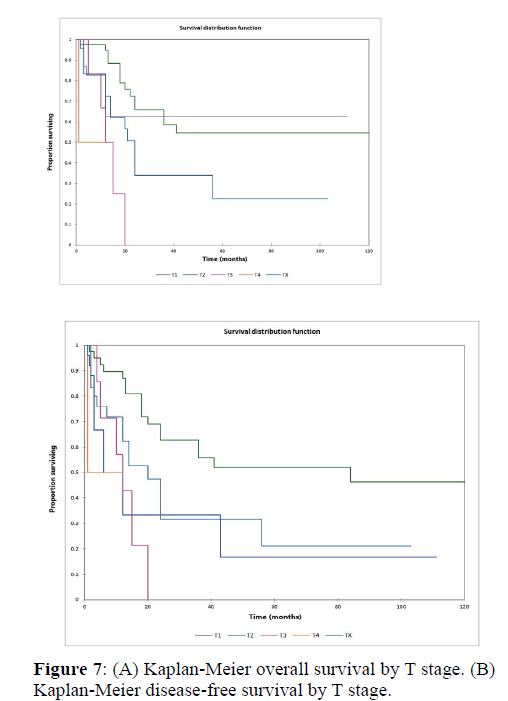
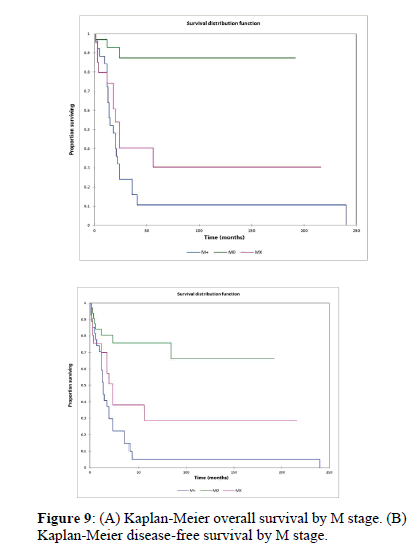
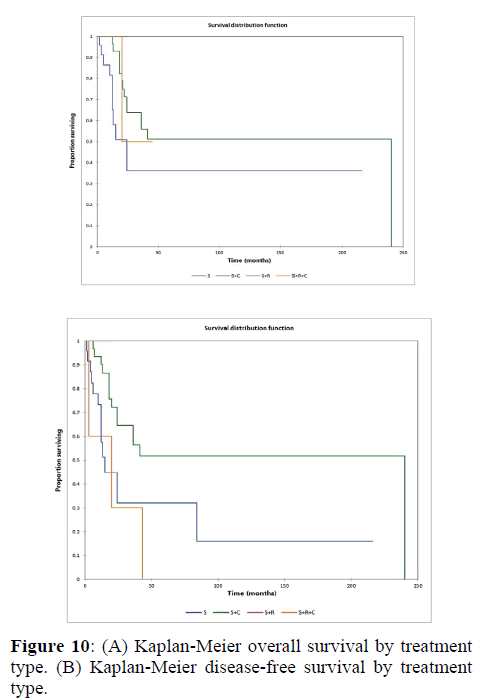
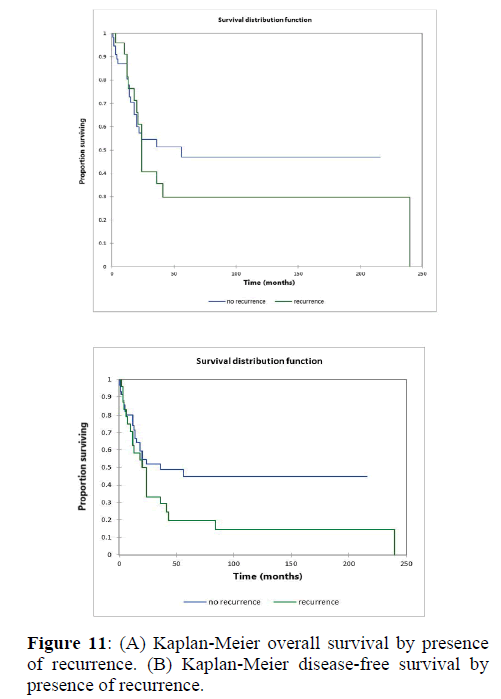
Figure 2a illustrates the Kaplan Meier actuarial overall survival (OS) for the entire cohort, while Figure 2b illustrates the Kaplan Meier actuarial disease-free survival (DFS) for the entire cohort. Overall 5-, 10-, and 20-year survival was 40.7, 40.7, and 40.7% respectively, while DFS at 5-, 10-, and 20-years was 35.1%, 31.9%, and 31.9%, respectively. Figure 3a illustrates the Kaplan Meier actuarial OS by histopathological subtype, while Figure 3b illustrates the Kaplan Meier actuarial DFS by histopathological subtype. Survival was greater for GCR osteosarcoma, GCR intraskeletal osteosarcoma (ESOS), and GCR malignant fibrous histiocytoma (MFH) and lower for GCR angiosarcoma, GCR undifferentiated carcinoma of the urinary tract, and GCR endometrioid adenocarcinoma (p<0.0001 for OS, p<0.0001 for DFS). Figure 4a illustrates the Kaplan Meier actuarial OS by primary site, while Figure 4b illustrates the Kaplan Meier actuarial DFS by primary site. Survival was greater for head and neck and limb primary sites, and lower for organ/soft tissue NOS (not otherwise specified), gastrointestinal/intraabdominal, and genitourinary primary sites (p<0.0001 for OS, p<0.0001 for DFS). Figure 5a illustrates the Kaplan Meier actuarial OS by overall American Joint Commission on Cancer (AJCC) stage, while Figure 5b illustrates the Kaplan Meier actuarial DFS by AJCC stage. OS was greater for stage I and II tumors than for stage III and IV tumors (p<0.0001 for OS), and DFS was greater for stage II tumors than for stage III and IV tumors (p<0.0001 for DFS). Figure 6a illustrates the Kaplan Meier actuarial OS by sex, while Figure 6b illustrates the Kaplan Meier actuarial DFS by sex. There was no significant difference in OS or DFS for male or female patients (p=0.5 for OS, p=0.9 for DFS). Figure 7a illustrates the Kaplan Meier actuarial OS by T stage, while Figure 7b illustrates the Kaplan Meier actuarial DFS by T stage. OS was greater for T1 and T2 tumors than for TX, T3, and T4 tumors, while DFS was greater for T2 tumors than TX, T3, and T4 tumors (p=0.002 for OS, p=0.005 for DFS). Figure 8a illustrates the Kaplan Meier actuarial OS by N stage, while Figure 8b illustrates the Kaplan Meier actuarial DFS by N stage. OS and DFS were greater for N0 patients than for NX and N+ patients (p=0.001 for OS, p=0.01 for DFS). Figure 9a illustrates the Kaplan Meier actuarial OS by M stage, while Figure 9b illustrates the Kaplan Meier actuarial DFS by M stage. OS and DFS were greater for M0 patients than for MX and M+ patients (p<0.0001 for OS, p<0.0001 for DFS). Figure 10a illustrates the Kaplan Meier actuarial OS by treatment type, while Figure 10b illustrates the Kaplan Meier actuarial DFS by treatment type. OS and DFS were greater for patients treated with surgery + chemotherapy (S+C) than for patients treated with surgery alone (S), (p<0.0001 for OS, p<0.0001 for DFS). Mean disease-free survival time was 20.0 months for S+R+C, 24.0 months for S+R, 134.4 months for S+C, 28.0 months for S alone, and 3.0 months for chemotherapy (C) alone. Mean overall survival time was 20.1 months for S+R+C, 24.0 months for S+R, 134.9 months for S+C, 34.9 months for S alone, and 4.0 months for chemotherapy (C) alone. Figure 11a illustrates the Kaplan Meier actuarial OS for patients who experienced recurrence and for patients who did not have recurrent disease, while Figure 11b illustrates the Kaplan Meier actuarial DFS for patients who experienced recurrence and for patients who did not have recurrent disease. While the decreased DFS seen in patients with recurrent disease approached survival, the OS and DFS were not statistically significantly different between the recurrence and no recurrence groups (p=0.5 for OS, p=0.07 for DFS).
Discussion
Giant cell rich cancers comprise a histologically diverse group of malignancies. While relatively rare, these cancers can affect primary sites encompassing the entire human body. Since Flanagan and coworkers [2] noted that the giant cells seen in the giant cell rich variant of MFH and giant cell granuloma were osteoclast-type cells, these osteoclastic giant cells have been noted in many other rare benign and malignant neoplasms and inflammatory lesions. Scotto di Carlo et al. [5] have found that mutations in the Histone H3. 3-protein encoding H3F3A gene may be implicated in the parthenogenesis of giant cell tumors in the clivus as well as in long bones. Histones are basic nuclear proteins that are responsible for the nucleosome structure of the chromosomal fiber in eukaryotes. Makise et al. [6] noted that among extraskeletal osteosarcomas (ESOS) the giant cellrich variant may show an H3K27me3 (the trimethylated lysine residue at position 27 in the protein histone H3) mutation. They noted that in addition to the genetic variations between the non-giant cell and giant cell rich versions of ESOS, that there may be survival differences, with non-giant cell ESOS in their series living from 3 to 103 months, while 2/3 of the H3K27me3-deficient giant cell-rich ESOS died from their disease in 4 and 20 months. Osteoclast-like giant cells have also been noted in urinary tract malignancy and malignant melanoma. [7,10,18] Amary et al. [46] noted that H3.3 mutations represented a reliable genetic marker for malignant giant cell rich of bone.
To our knowledge the present study is the first meta-analysis of giant cell malignancies. Univariate analysis found histopathological type, primary site, AJCC stage, T, N, and M stage, and treatment type to significantly affect overall and disease-free survival, while multivariate analysis found that histopathology alone significantly affected overall and diseasefree survival. Patients with osteosarcoma or malignant fibrous histiocytoma histologic type, head and neck or limb primary site, and lower AJCC, T, N, and M stage had improved survival. Patients treated with surgery + chemotherapy appeared to have significantly improved survival over patients treated with single modality surgery alone or chemotherapy alone treatment, and improved survival over patients treated with surgery, radiation, and chemotherapy or surgery + radiation. The improved survival in patients treated with surgery + chemotherapy is likely a result of the large percentage of giant cell rich osteosarcoma and malignant fibrous histiocytoma patients in the cohort, as these sarcomas tend to respond well to surgery and chemotherapy. [50] The decreased survival in patients treated with surgery + radiation + chemotherapy may reflect the relatively low number of patients in the S+R+C group (6), and the fact that 1/3 of these were stage IV. Nonetheless combined modality therapy in general appears to offer better survival outcomes than single modality therapy such as surgery alone or chemotherapy alone in giant-cell rich malignancies, although larger studies would be needed to conform these results.
This study demonstrated an overall 5-year survival of approximately 40% and a disease-free 5 year survival of approximately 35% for the entire cohort. The most frequent tumors found in the cohort, giant cell rich osteosarcoma and giant cell rich malignant fibrous histiocytoma, had 5-year overall survival rates of approximately 55% and 50%, respectively and 5-year disease-free survival rates of approximately 50% and 45%. In their pooled analysis study of survival in osteosarcoma in patients treated with surgery and intensive or conventional chemotherapy, Zhang et al. [51] noted a pooled 5-year overall survival rate of 63.1% in the intensified chemotherapy group, and 61.9% in the conventional chemotherapy group. In their 11-year study of survival outcomes in head and neck sarcoma, of which malignant fibrous histiocytoma was one of the largest groups, Sharma et al. [52] noted an overall 5-year survival of 82.7% while 5-year disease-free survival was 55.3%. Ozkurt et al. [53] noted a 5-year survival rate of 81.9% in patients with wide surgical margins and 33.3% in patients with marginal margins in their study of long-term follow up in patients treated for malignant fibrous histiocytoma of the long bones. The lower 5-year overall and disease-free survival in the present giant-cell rich group in the present study vs. those of a crosssection of more conventional sarcomas in the aforementioned studies points to a likely lower survival rate for the giant-cell rich variants of the tumors in the present study. The giant cell variants of the malignancies noted in the present study may behave in a more aggressive fashion vs. their non-giant cell rich variants, resulting in the relatively low overall and diseasefree survival noted in the giant cell rich malignancies seen in the present study. Futures studies may provide insight into the nature the giant cells present in these malignancies play in their development, growth, and metastasis. The giant cell rich variant of these malignancies may also exhibit more unpredictable clinical behavior.
This study has several limitations, including the relatively low patient numbers for this rare group of malignancies, and the diverse collection of primary sites and histopathological types, each with their own AJCC staging systems. The relatively low numbers of giant cell rich malignancies reported in the literature necessitated the inclusion of the diverse group of histopathological subtypes for analysis. Futures studies may be able to draw upon larger numbers of single histopathological subtypes. Additionally, the retrospective nature of the data makes recall and selection bias a possibility.
Conclusion
Giant-cell rich malignancies are a diverse group of cancers that are relatively rare. This meta-analysis found that primary site, histopathological type, treatment type, and AJCC, T, N, and M stage all significantly affected overall and disease-free survival on univariate analysis. Multimodality therapy including surgical resection when possible appears to confer the best survival outcomes. These malignancies tend to be aggressive, and 13.4% of patients had nodal metastases and 32.9% had distant metastases, and five-year overall and disease-free survival was less than 50% for the cohort. Further studies with larger patient numbers may further illuminate optimal treatment regimens.
Conflict of Interest
The authors disclose that they have no conflicts of interest.
REFERENCES
- Flanagan AM, Nui B, Tinkler SM, Horton MA, Williams DM, Chambers TJ. The multinucleate cells in giant cell granulomas of the jaw are osteoclasts. Cancer. 1988;62:1139-1145.
- Flanagan AM, Chambers TJ. Osteoclasts are present in the giant cell variant of malignant fibrous histiocytoma. J Pathol. 1989;159:53-57.
- Macagno N, Caselles K, Aubert S, Audard V, Gomez-Brouchet A, Galant C, et al. Benign and malignant giant-cell rich lesions of bone: Pathological diagnosis with special emphasis on recent immunohistochemistry and molecular techniques. Ann Pathol. 2018;38:92-102.
- Kumar R, Bharani V, Gupta N, Gupta K, Dey P, Srinivasan R, et al. Giant cell tumour of tendon sheath: A 10-year study from a tertiary care centre. Cytopathology. 2018;29:288-293.
- Scotto di Carlo F, Divisato G, Iacoangeli M, Esposito T, Gianfrancesco F. The identification of H3F3A mutation in giant cell tumour of the clivus and the histological diagnostic algorithm of other clival lesions permit the differential diagnosis in this location. BMC Cancer. 2018;18:358.
- Makise N, Sekimizu M, Kubo T, Wakai S, Watanabe SI, Kato T, et al. Extraskeletal osteosarcoma: MDM2 and H3K27me3 analysis of 19 cases suggest disease heterogeneity. Histopathology. 2018.
- Jiménez-Heffernan JA, Adrados M, Muñoz-Hernández P, Fernández-Rico P, Ballesteros-García AI, Fraga J. Cytologic features of malignant melanoma with osteoclast-like giant cells. Acta Cytol. 2018;62:151-154.
- Choo JY, Lee JH, Lee JY, Park YM. Cutaneous metastasis of giant cell-rich osteosarcoma. Ann Dermatol. 2016;28:247-248.
- Chow LT. Giant cell rich osteosarcoma revisited-diagnostic criteria and histopathologic patterns, Ki67, CDK4, and MDM2 expression, changes in response to bisphosphonate and denosumab treatment. Virchows Arch. 2016;468:741-755.
- Nawar NA, Olsen J, Jelic TM, He C. Primary urinary bladder angiosarcoma with osteoclast-like multinucleated giant cells: A case report and literature review. Am J Case Rep. 2016;17:143-149.
- Vijayan S, Naik MA, Hameed SA, Rao SK. Giant cell rich osteosarcoma of the cuneiforms. J Cancer Res Ther. 2015;11:989-992.
- Ahrari A, Labib M, Gravel D, Macdonald KI. Primary osteosarcoma of the skull base treated with endoscopic endonasal approach: A case report and literature review. J Neurol Surg Rep. 2015;76:e270-e274.
- Sun LM, Zhang QF, Tang N, Mi XY, Qiu XS. Giant cell rich osteosarcoma of the mandible with abundant spindle cells and osteoclast-like giant cells mimicking malignancy in giant cell tumor. Int J Clin Exp Pathol. 2015;8:9718-9722.
- Chow LT. Fibular giant cell-rich osteosarcoma virtually indistinguishable radiographically and histopathologically from giant cell tumor-analysis of subtle differentiating features. APMIS. 2015;123:530-539.
- Alemán-Meza L, Gómez-Macías GS, Barboza-Quintana O, Garza-Guajardo R, Loya-Solis A. Osteoclastic giant cell rich squamous cell carcinoma of the uterine cervix: A case report and review of the literature. Case Rep Pathol. 2014;2014:415328.
- Chow LT, Wong SK. Epiphyseal osteosarcoma revisited: four illustrative cases with unusual histopathology and literature review. APMIS. 2015;123:9-17.
- Huang J, Jiang Z, Zhang H. Clinicopathologic differential diagnosis of giant cell-rich osteosarcoma and giant cell tumor of bone. Zhonghua Bing Li Xue Za Zhi. 2014;43:379-382
- Mallick S, Singh L, Rajan K, Sharma MC, Bansl V, Dinda AK. Malignant melanoma of soft parts with osteoclast-rich giant cells: A rare tumour of the jejunum. Australas Med J. 2014;7:181-184.
- Knösel T, Werner M, Jung A, Kirchner T, Dürr HR. Dedifferentiated chondrosarcoma mimicking a giant cell tumor. Is this low grade dedifferentiated chondrosarcoma? Pathol Res Pract. 2014;210:194-197.
- Ishida M, Okabe H. Endometrioid adenocarcinoma with choriocarcinomatous differentiation: A case report and review of the literature. Oncol Lett. 2013;6:655-658.
- Calli AO, Tunakan M, Katilmis H, Kilçiksiz S, Oztürkcan S. Soft tissue giant cell tumor of low malignant potential of the neck: a case report and review of the literature. Turk Patoloji Derg. 2014;30:73-77.
- Wang CS, Yin QH, Liao JS, Lou JH, Ding XY, Zhu YB. Giant cell-rich osteosarcoma in long bones: clinical, radiological and pathological features. Radiol Med. 2013;118:1324-1334.
- Momin YA, Bharambe BM, Ansari SA, Ghodke BA. Osteoclastic giant cell rich metaplastic carcinoma in malignant phylloides: A rare entity. South Asian J Cancer. 2012;1:93-95.
- Huang J, Jiang Z, Yang Q, Zhang H. Benign looking giant cell component in dedifferentiated chondrosarcoma: benign or malignant? A case report. Int J Surg Pathol. 2013;21:48-53.
- Vijay MK, Das P, Prabhu SB, Mouli P, Acharya SK, Mathur SR. Pleomorphic giant cell-rich hepatocellular carcinoma presented as a right atrial mass. Indian J Pathol Microbiol. 2011;54:632-634.
- Insabato L, Masone S, Campione S, Vigliar E, Staibano S, Tornillo L. Coexistence of primary gastric giant cell-rich leiomyosarcoma and gastrointestinal stromal tumor: A report of a very rare combination and review of the literature. Int J Surg Pathol. 2012;20:74-78.
- Fu HH, Zhuang QW, He J, Wang LZ, He Y. Giant cell-rich osteosarcoma or giant cell reparative granuloma of the mandible? J Craniofac Surg. 2011;22:1136-1139.
- Bui MM, Purohit CN, Hakam A. Fine needle aspiration biopsy of an osteoclast-rich undifferentiated urothelial carcinoma: A cytology case report and review of the literature. Cytojournal. 2010;7:18.
- Serin G, Doganavsargil B, Caliskan C, Akalin T, Sezak M, Tunçyürek M. Colonic malignant melanoma, primary or metastatic? Case report. Turk J Gastroenterol. 2010;21:45-49.
- Jadhav DS, Bagate AN, Swami SY, Sonwane BR. Metaplastic carcinoma of breast-giant cell rich variant. Indian J Cancer. 2010;47:88-89.
- Brownlow HC, Ioannidis G, Gibbons MC, Jones AC, Athanasou N. Digital metastases of giant cell rich malignant fibrous histiocytoma. Sarcoma. 1999;3:167-170.
- Kinoshita G, Yasoshima H. Giant cell-rich tumor of the rib. J Orthop Sci. 2006;11:312-317.
- Bertoni F, Bacchini P, Staals EL, Davidovitz P. Dedifferentiated parosteal osteosarcoma: the experience of the Rizzoli Institute. Cancer. 2005;103:2373-2382.
- Bertoni F, Bacchini P, Staals EL. Malignancy in giant cell tumor. Skeletal Radiol. 2003;32:143-146.
- Bertoni F, Bacchini P, Staals EL. Giant cell-rich osteosarcoma. Orthopedics. 2003;26:179-181.
- Leonard J, Gökden M, Kyriakos M, Derdeyn CP, Rich KM. Malignant giant-cell tumor of the parietal bone: case report and review of the literature. Neurosurgery. 2001;48:424-429.
- Mori Y, Tsuchiya H, Karita M, Nonomura A, Nojima T, Tomita K. Malignant transformation of a giant cell tumor 25 years after initial treatment. Clin Orthop Relat Res. 2000;381:185-191.
- Asano K, Sekiya T, Shimamura N, Tanaka M, Takemura A, Suzuki S, et al. A case of giant cell-rich gliosarcoma. No To Shinkei. 1997;49:938-944.
- Val-Bernal JF, Corral J, Fernández F, Gómez-Bellvert C. Atypical fibroxanthoma with osteoclast-like giant cells. Acta Derm Venereol. 1994;74:467-470.
- Mentzel T, Fletcher CD. Malignant mesenchymomas of soft tissue associated with numerous osteoclast-like giant cells mimicking the so-called giant cell variant of "malignant fibrous histiocytoma". Virchows Arch. 1994;424:539-545.
- Ohara N, Hayashi K, Shinohara C, Kamitani M, Furuta T, Yoshino T, et al. Primary osteosarcoma of the cerebrum with immunohistochemical and ultrastructural studies: A report of a case. Acta Neuropathol. 1994;88:384-388.
- Ito Y, Sugita S, Segawa K, Sugawara T, Tsujiwaki M, Fujita H, et al. Giant cell-rich osteosarcoma of the vertebra with murine double minute chromosome 2- and cyclin-dependent kinase 4-positive and histone H3F3A mutant p.Gly34Trp-negative immunophenotypes. Pathol Int. 2018;68:324-326.
- Priore SF, Schwartz LE, Epstein JI. An expanded immunohistochemical profile of osteoclast-rich undifferentiated carcinoma of the urinary tract. Mod Pathol. 2018.
- Righi A, Mancini I, Gambarotti M, Picci P, Gamberi G, Marraccini C, et al. Histone 3.3 mutations in giant cell tumor and giant cell-rich sarcomas of bone. Hum Pathol. 2017;68:128-135.
- Zambo I, Pazourek L. Giant cell-rich lesions of bone and their differential diagnosis. Cesk Patol. 2017;53:61-70.
- Amary F, Berisha F, Ye H, Gupta M, Gutteridge A, Baumhoer D, et al. H3F3A (Histone 3.3) G34W Immunohistochemistry: A reliable marker defining benign and malignant giant cell tumor of bone. Am J Surg Pathol. 2017;41:1059-1068.
- Hirose K, Okura M, Sato S, Murakami S, Ikeda JI, Noda Y, et al. Gnathic giant-cell-rich conventional osteosarcoma with MDM2 and CDK4 gene amplification. Histopathology. 2017;70:1171-1173.
- Huang EC, Ghazikhanian V, Qian X. Giant cell-rich osteosarcoma of the parotid gland: An exceptionally rare entity at an unusual site. Diagn Cytopathol. 2016;44:1107-1111.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006-1012.
- Gore MR. Treatment, outcomes, and demographics in sinonasal sarcoma: a systematic review of the literature. BMC Ear Nose Throat Disord. 2018;18:4.
- Zhang Y, He Z, Duan Y, Wang C, Kamar S, Shi X, et al. Does intensified chemotherapy increase survival outcomes of osteosarcoma patients? A meta-analysis. J Bone Oncol. 2018;12:54-60.
- Sharma N, George NA, Singh R, Iype EM, Varghese BT, Thomas S. Surgical management of head and neck soft tissue sarcoma: A 11-year experience at a tertiary care centre in South India. Indian J Surg Oncol. 2018;9:187-191.
- Özkurt B, Basarir K, Yildiz YH, Kalem M, Saglik Y. Primary malignant fibrous histiocytoma of long bones: Long-term follow-up. Eklem Hastalik Cerrahisi. 2016;27:94-99.





 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.