Hormonal Changes in Women with Polycystic Ovary Syndrome in Relation with BMI
Received: 15-May-2023, Manuscript No. AMHSR-23-98861; Editor assigned: 18-May-2023, Pre QC No. AMHSR-23-98861 (PQ); Reviewed: 01-Jun-2023 QC No. AMHSR-23-98861; Revised: 14-Jul-2023, Manuscript No. AMHSR-23-98861 (R); Published: 21-Jul-2023
Citation: Suhas P. Hormonal Changes in Women with Polycystic Ovary Syndrome in Relation with BMI. Ann Med Health Sci Res. 2023;13:882-884
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Polycystic Ovary Syndrome (PCOS) is a common heterogeneous disorder in women of reproductive age and the most frequent cause of hyperandrogenism, combined with anovulatory infertility. Its complex pathogenesis involves hypothalamic-pituitary disturbances in gonadotropin secretion, speci ically increased levels of Luteinizing Hormone (LH) with impaired ovary steroidogenesis and reducing the Synthesis of gender Hormone-Binding Globulin (SHBG). A case control study was conducted with 100 diagnosed PCOS patients (50 obese and 50 non-obese) and 100 controls (50 obese and 50 non-obese) aged 18-40 years at Koppal institute of medical sciences, Koppal. All the data were expressed as mean ± SE. The mean were analyzed by one way ANOVA followed by student Newman Keul’s multiple comparison tests. LH, Testosterone, estrogen, AMH and DHEAS were increased while FSH and SHBG were decreased irrespective of BMI. However Prolactin (PRL) did not show any statistical signi icance. Hormonal disturbances are due to hypothalamic-pituitary axis abnormality with increased gonadotropin secretion, ovary steroidogenesis and reducing the synthesis of SHBG irrespective of BMI status of women with PCOS.
Keywords
PCOS; LH; Testosterone; Estrogen; AMH; FSH; SHBG
Introduction
Polycystic Ovary Syndrome (PCOS) is a common heterogeneous disorder in women of reproductive age and the most frequent cause of hyperandrogenism, combined with an ovulatory infertility. It is a complex and multifactorial disorder where the signs and symptoms vary among each individual woman depending on their hormonal and biochemical differences. Its complex pathogenesis involves.
• Hypothalamic-pituitary disturbances in gonadotropin
secretion, specifically increased LH levels.
• Impaired ovary steroidogenesis.
• Insulin Resistance (IR) resulting in compensatory
hyperinsulinemia, which contributes to PCOS, associated
hyper-androgenism by enhancing androgen production and
reducing the Synthesis of gender Hormone-Binding
Globulin (SHBG).
The risk of type 2 diabetes mellitus among PCOS patients is 5-10 fold higher than normal [1,2]. Insulin has direct and indirect roles in the pathogenesis of hyperandrogenism in PCOS. Insulin in collaboration with Luteinising Hormone (LH) enhances the androgen production of theca cells. IR is found in both lean and obese women with PCOS, but obesity and PCOS independently may affect IR [4]. This syndrome is thought to affect women throughout their life specifically on their gynecological and metabolic status. Hence it is extremely important to diagnose polycystic ovary syndrome women at an early age to avoid long term complications. The biochemical and hormonal alterations are thought to be the major modifying factor. Most of the studies available are in obese individuals hence we conducted the study to compare the differences in the obese and non-obese PCOS women with respect to their controls in terms of endocrine hormones in South Indian women.
Case Presentation
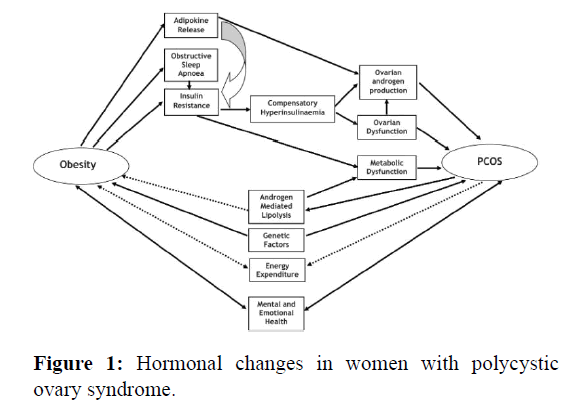
A case-control study was conducted on 100 diagnosed PCOS patients (50 obese and 50 non-obese) and 100 controls (50 obese and 50 non-obese) aged 18-40 years at Koppal institute of medical sciences, Koppal. Women under the age of 18 years and women with inflammatory conditions, congenital adrenal hyperplasia, hyperthyroidism, hypothyroidism, goiter, hyperprolactinaemia, cushing’s syndrome and on any drug therapy were excluded from the study. Ethical committee approval was obtained by the institutional ethics committee. Informed consent was obtained from the participants. A physical examination of each subject was carried out. The height and weight of all individuals were measured. Body Mass Index (BMI) was calculated by kg/m2. The diagnosis of PCOS was done according to the Rotterdam ESHRE revised consensus 2003. The Rotterdam criterion is presently used for diagnosing polycystic ovarian syndrome as it is the most widely accepted criteria. Fasting blood samples of 10.0 ml was obtained by venipuncture from each subject who participated in the study. The samples were centrifuged at 3000 rpm for 10 mins at 200℃ to separate the serum/ plasma and it was stored at -200℃ until the tests were performed. All hormones were evaluated using the chemiluminometric method (Figure 1).
Results and Discussion
Although, PCOS is an ovarian disease it interacts with one or more different congenital and perhaps environmental factors leading to the deregulation of steroidogenesis [3] and hyperandrogenism [4]. Elevated androgens in PCOS is due to increased secretion of androgens from ovaries (25% to 30%), adrenals and peripheral steroid conversion to androgen as well as the ability of human adipose tissue to synthesize androgens [5-8]. Upper body weight and visceral fat are important contributors. In our study, we observed increased levels of LH, estrogen, testosterone, AMH, DHEAS and decreased levels of FSH and SHBG, irrespective of BMI which explains the cause for an ovulatory cycles and infertility. Increased testosterone production by theca cells of women with PCOS was also observed by Nestler JE, et al. [9]. Suppression of hepatic production of Gender Hormone Binding Globulin (GHBG), leads to an increase in testosterone that interacts with the granulosa cells causing abnormal differentiation and premature arrest of follicular growth, and thus anovulation. Hyperinsulinemia also intensifies the response of granulosa cells to LH. Similar observations were made in other studies [10]. Similar observations of higher LH pulse and amplitude in women with PCOS [11,12].
Conclusion
Increased levels of LH and decreased FSH leading to failure of follicular development and thereby decreased production of progesterone and estradiol manifest as anovulation in PCOS irrespective of BMI. Increased levels of testosterone and decreased levels of SHBG lead to hyper androgen status in women with PCOS irrespective of BMI. Increased levels of estrogen, DHEAS and AMH may also be held responsible for adding on to various signs and symptoms in women with PCOS irrespective of BMI.
References
- Khan KA, Stas S, Kurukulasuriya LR. Polycystic ovarian syndrome. J Cardiometab Syndr. 2007;1:125-130.
[Crossref] [Google Scholar] [PubMed]
- Azziz A, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, et al. Androgen excess in women: Experience with over 1000 consecutive patients. J Clin Endocrinol Metabol. 2004;89:453-462.
[Crossref] [Google Scholar] [PubMed]
- Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223-1236.
[Crossref] [Google Scholar] [PubMed]
- Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;1165-11674.
[Crossref] [Google Scholar] [PubMed]
- Piltonen T, Morin-Papunen L, Koivunen R, Perheentupa A, Ruokonen A, Tapanainen JS. Serum anti-mullerian hormone levels remain high until late reproductive age and decrease during metformin therapy in women with polycystic ovary syndrome. Hum Reprod. 2005;20:1820-1826.
[Crossref] [Google Scholar] [PubMed]
- Franks S, Gilling SC, Watson H, Willis D. Insulin action in the normal and polycystic ovary. Endocrinol Metab Clin North Am. 1999;28:361-378.
[Crossref] [Google Scholar] [PubMed]
- Fassnacht M, Schlenz N, Schneider SB, Wudy SA, Allolio B, Arlt W. Beyond adrenal and ovarian androgen generation: Increased peripheral 5 alpha-reductase activity in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:2760-2766.
[Crossref] [Google Scholar] [PubMed]
- Quinkler M, Sinha B, Tomlinson JW, Bujalska IJ, Stewart PM, Arlt W. Androgen generation in adipose tissue in women with simple obesity-a site specific role for 17 beta-hydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183:331-342.
[Crossref] [Google Scholar] [PubMed]
- Nestler JE, Jakubowicz DJ, de Vargas AF. Insulin stimulates testosterone biosynthesis by human thecal cells from women with polycystic ovary syndrome by activating its own receptor and using inositol glycan mediators as the signal transduction system. J Clin Endocrinol Metab. 1998;83:2001-2005.
[Crossref] [Google Scholar] [PubMed]
- Clayton RN, Ogden V, Hodgkinson J. How common are polycystic ovaries in normal women and what is their significance for the fertility of the population? Clin Endocrinol. 1992;37:1271-1234.
[Crossref] [Google Scholar] [PubMed]
- Waldstreicher J, Santoro NF, Hall HE. Hyperfunction of the hypothalamic-pituitary axis in women with polycystic ovarian disease: Indirect evidence for partial gonadotroph desensitization. J Clin Endocrinol Metab. 1988;66:165-172.
[Crossref] [Google Scholar] [PubMed]
- Morales AJ, Laughlin GA, Butzow T, Maheshwari H, Baumann G, Yen SS. Insulin, somatotropic, and luteinizing hormone axes in lean and obese women with polycystic ovary syndrome: Common and distinct features. J Clin Endocrinol Metab. 1996;81:2854-2864.
[Crossref] [Google Scholar] [PubMed]




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.