Immunohistochemical Assessment of Proliferating Cell Nuclear Antigen Protein Expression in Plaque, Reticular and Erosive Types of Oral Lichen Planus
- *Corresponding Author:
- Dr. Suresh KV
Department of Oral Medicine and Radiology, School of Dental Sciences Krishna Institute of Medical Sciences, Deemed University, Karad, Satara, Maharashtra, India.
E-mail: dr.suri88@gmail.com
Abstract
Background: Proliferating cell nuclear antigen (PCNA) is a nuclear protein synthesized in the late G1 and S‑phase of the cell cycle. Immunodetection of this protein represents a useful marker of the proliferation status of lesions. Aims: The aim of this study is to evaluate the immunohistochemical expression of PCNA in oral lichen planus (OLP) and to assess the PCNA expression in a different layer of epithelium in different types of OLP. Subjects and Methods: A total of 96 cases of histologically proven OLP, 32 cases each of erosive, reticular and plaque type were selected. Two sections were taken from each one for H and E. Other sections were stained according to super sensitive polymer horseradish peroxidase method for identifying PCNA expression. Results: Of the three types of OLP, erosive type showed higher expression of PCNA (average 66.8%, minimum of 55% and maximum of 80.3%) followed by reticular (average 37.7%, minimum of 26% and maximum of 47%) and plaque type (average 17%, minimum of 5% and maximum of 25%) indicating increased proliferative activity. The erosive type also showed higher expression of PCNA in all the layers of epithelium followed by reticular and plaque type. Conclusion: PCNA is a good marker to indicate proliferation status of disease. Out of three types, erosive type possess more proliferative ratio, chances of malignant changes is more in this type.
Keywords
Immunohistochemistry, Oral lichen planus, Proliferating cell nuclear antigen
Introduction
Oral lichen planus (OLP) is the most common mucocutaneous condition presenting in the oral cavity.[1] This condition was first described by Wilson in 1869. OLP affects 0.1-4% of the world’s population and 1.5% of Indian population. It is predominantly found in the middle age, but occasionally children are also affected.[2,3]
OLP has been categorized into several clinical forms. The most common is the reticular type, which present as white striae known as “Wickham’s striae.” Patients with reticular lesions are often bilateral and asymptomatic. Plaque type of OLP appears as a homogenous white patch resembles leukoplakia. This form may range from slightly elevated, smooth lesions to slightly irregular lesions, which may be multifocal. Erosive OLP presents as irregular erosion or ulceration covered with a fibrinous plaque. The periphery of the lesion is usually surrounded by reticular or finely radiating keratotic striae. It is often associated with a burning sensation and pain and it is exacerbated by trauma and food particularly hot, spicy and acidic foods.[1,2]
Malignant transformation of OLP has become much of controversy when the first case of gingival cancer was reported in a patient with OLP in 1910. Few studies have suggested that OLP has increased malignant transformation, based on these studies the World Health Organization has classified OLP as a potentially malignant disease.[4] Some authors, however, argued that such transformation has not been sufficiently documented to justify this classification. According to these authors, more precise criteria are needed to diagnose OLP, especially from a histopathological standpoint.
In several types of oral cancer, the evaluation of the cell proliferation rate brings important information regarding diagnosis and prognosis. The enhancement of the proliferation capacity may be one of the first indicators of malignant transformation since it constitutes a key event for the development of cancer.[5,6] The true malignant transformation of OLP can be evaluated by analyzing the expression proteins related to cell proliferation and apoptosis as alterations in these proteins are essential for carcinogenesis.[7-9] Proliferating cell nuclear antigen (PCNA) is a useful protein marker to assess proliferation status of lesions. Higher the cell proliferation rate, the higher risk of malignant transformation. In this context, OLP with increased PCNA can have a higher malignant transformation risk.[1]
Subjects and Methods
Study design
On a total of 96 cases of previously diagnosed OLP of buccal mucosa, 32 cases of each plaque, reticular and erosive were collected. Cases of lichenoid dysplasia and lichenoid reaction were excluded from the study. In our study, subjects were considered after clinical and histopathological diagnosis and before management as treatment by topical steroids would alter the pathophysiology and thus significantly alter the results of this study. All subjects gave their informed consent and ethical clearance was obtained from local ethical committee. Histological sections were prepared from paraffin embedded blocks. One section was stained with Hematotoxylin and Eosin to verify histological diagnosis according to Eisenberg criteria. Other sections were stained according to super sensitive polymer horse radish peroxidise (HRP) method for identifying immunohistochemical expression of PCNA. Sections cut at four microns were floated on to Poly-L-Lysine coated slides and incubated overnight at 58°C. The sections were then deparaffinized in two changes of xylene for 15 min each. Dexylinization was performed by immersing the slides in two changes of absolute alcohol for 1 min each. Sections were alcoholized by immersing the slides in 90% and 70% alcohol for 1 min each and then washed for 10 min and 5 min each in tap water and distilled water respectively. Antigen retrieval was carried out by placing the sections in citrate buffer and then pressure cookerizing for 10 min. Pressure cooker was then cooled for 20 min in the sink with water. Sections were then rinsed with distilled water for 5 min and were then washed with two changes of tris buffer solution (TBS) for 5 min each. To block the endogeneous peroxidase enzyme activity, the sections were treated with peroxidase block for 10-15 min and then again washed with three changes of TBS for 5 min each. Sections were then treated with power block for 15 min in order to block non-specific reaction with other antigens. Sections were then drained and covered with primary antibody against PCNA with dilution of 1:100 for 1 h to identify tumor markers by antigen-antibody reactions and again washed with TBS as described earlier. To enhance the reaction between primary and secondary antibodies, sections were then treated with super enhancer for 30 min, again washed with TBS. Enzymes were labeled by treating the sections with super sensitive poly-HRP secondary antibody and washed with TBS. Chromogen was then added to the sections for 5 min to give color to the antigens and sections were again washed with TBS. Sections were then washed with tap water for 5 min and were counterstained with Hemotoxylin for 1 min and washed in tap water, dried, cleared in xylene and mounted with Di-n-butyl Phthalate in Xylene. Diagnosed lymphoma cases were taken as a positive control for PCNA expression and for negative control the primary antibody was omitted during the Immunohistochemical staining.[5]
Eisenberg (2000) histopathological criteria for the diagnosis of OLP, included as essential and non-essential findings.[10]
Essential findings are presence
• Liquefied baseline layer
• Intense lymphocyte infiltration in layers underlying the epithelium with effacement of the baseline layer
• Normal epithelial cell maturation.
Other findings (non-essential)
• Interpapillary crests in a “saw tooth” shape
• Hyperparakeratosis
• Civatte bodies
• Separation of the epithelium of the lamina propria.
Exclusion criteria
• Cells with large and/or hyperchromatic nuclei
• Presence of dyskeratosis
• Increased number of mitoses or atypical mitoses
• Projection of epithelial “drop-like” cones
• Absence of liquefied baseline layer
• Loss of epithelial stratification
• Heterogeneous inflammatory infiltrate
• Extension of infiltrate to deeper layers
• Perivascular infiltrate.
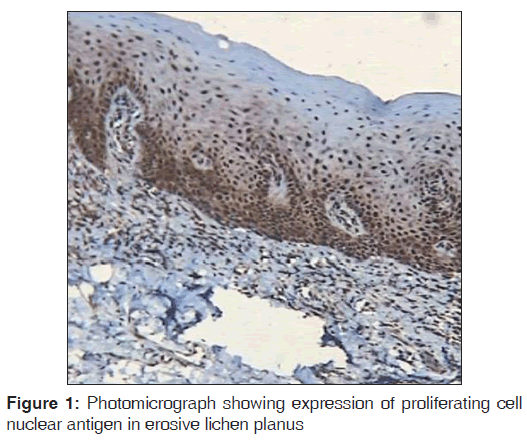
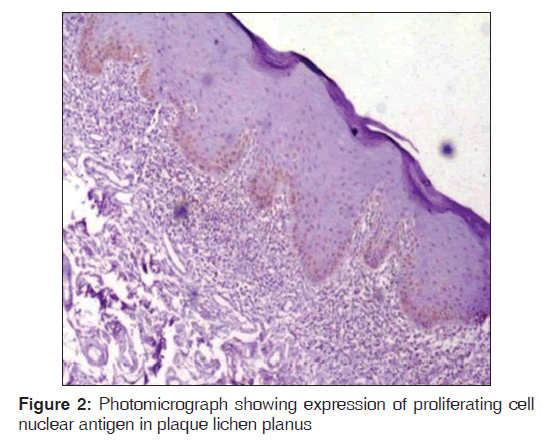
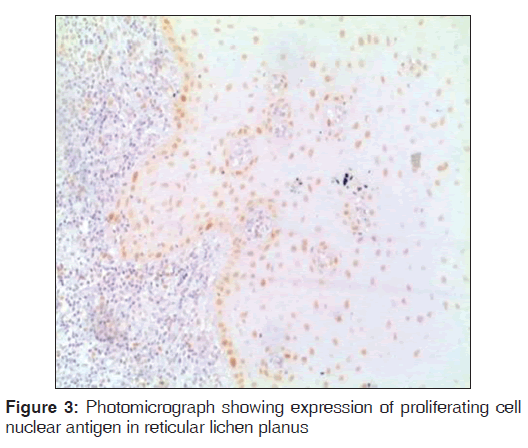
Regarding PCNA expression, the cases were classified according to the number of positively stained cells per 1000 counted cells in all epithelium layers (basal, intermediate and superficial layers) as: Positive (more than 5% of cells were stained) and negative (less than 5% of cell stained) [Figures 1-3].
In a slide 3-4 fields were selected and counted and data was statistically analyzed by Tukey honestly significant difference test. Overall data was found to be statistically significant.
Results
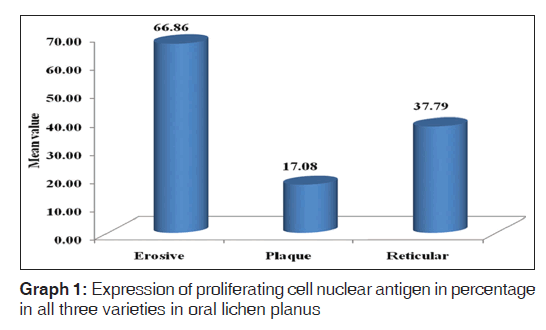
Out of the three varieties, erosive type (66.8%) showed higher expression of PCNA followed by reticular type (37.7%) and plaque (17%). It indicates proliferation activity was more in erosive lichen planus followed by reticular and plaque type. In erosive type, minimum expression was 55% and maximum expression was 80.3% standard deviation was 9.65. In reticular type minimum, PCNA expression was 26% and maximum expression was 47.3%, standard deviation found to be 8.17. In plaque variety, minimum expression of PCNA was 5.6% and maximum expression was 25.6% standard deviation found to be 6.3. Overall the values were found to be statistically significant [Table 1 and Graph 1]. When erosive type was compared with plaque type, mean difference was 49.7%, erosive type to reticular type was 29.% and plaque type to reticular type was –20.7% [Table 2].
| Types of OLP | N | Mean in% | Standard deviation in% | Minimum in% | Maximum in% |
|---|---|---|---|---|---|
| Erosive | 32 | 66.8 | 9.6 | 55 | 80.3 |
| Plaque | 32 | 17 | 6.3 | 5.6 | 25.6 |
| Reticular | 32 | 37.7 | 8.1 | 26 | 47.3 |
P<0.001. PCNA: Proliferating cell nuclear antigen, OLP: Oral lichen planus
Table 1: Expression of PCNA in percentage in all three varieties in OLP
| (I) Group to (J) Group | Mean difference (I-J) | P vhs |
|---|---|---|
| Erosive to plaque | 49.7 | <0.001 |
| Erosive to reticular | 29 | <0.001 |
| Plaque to reticular | −20.7 | <0.001 |
PCNA: Proliferating cell nuclear antigen, OLP: Oral lichen planus
Table 2: Multiple comparisons of PCNA expression in three types of OLP (in %)
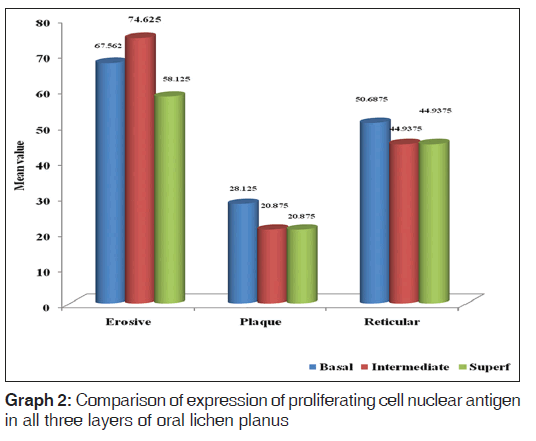
When basal, intermediate and superficial layers were compared in plaque type, reticular and erosive type of OLP, erosive type showed higher expression of PCNA in basal, intermediate and superficial layers followed by reticular and then plaque in all respective layers [Graph 2].
Discussion
Lichen planus is relatively common, chronic inflammatory mucocutaneous disease affecting the oral mucosa. The oral lesions of lichen planus shows different clinical patterns than those of cutaneous counterpart and are categorized as reticular, papular, plaque, atrophic, erosive, and bullous forms. Malignant transformation of OLP, especially the erosive variety, has been suggested, but the premalignant potential of lichen planus is still controversial.[2,11]
Histopathologically plaque type of OLP shows thick layer of hyperkeratosis and band like inflammatory cell infiltration in the lamina propria. Erosive type shows ulcerated and atrophic epithelium, a broad eosinophillic band is present below the covering epithelium.[12]
According to Mignogna et al., chronic inflammation in case of OLP, generates a cytokine-based micro environment that affects epithelial cell survival, growth, proliferation and differentiation; this may consequently contribute to cancer initiation, promotion and progression.[13]
PCNA is an enzyme related to the proliferative state of the cell because of its close association to components of the cell cycle. However, the PCNA expression may be associated with deoxyribonucleic acid (DNA) repair process or stimulated by such growth factors like cytokines. Because of its long half-life, PCNA may be detected in cells that have left the cell cycle.[14]
According to Mitamura et al., expression of PCNA is more in reticular and plaque type than atropic type and normal buccal mucosa.[11] In contrast to this, our study there was increased expression of PCNA in erosive type followed by reticular and plaque type. On the other hand Sousa et al.,[6] found that 58.33% of lichen planus were positive for PCNA and 83.33% were positive in epithelial dysplasia. This suggests that there is increased potential for malignant transformation in both lichen planus and epithelial dysplasia. In contrast to this, our study verified the expression of PCNA in different types of OLP. All types of OLP were positive for PCNA, out of the three types of OLP; erosive type has more potential for malignancy as compared with plaque and reticular types.
According to Lee et al., PCNA expression was higher in the atrophic form compared to the other forms, this suggests that the atrophic form has a higher malignant potential.[14] Da Silva Fonseca and do Carmo evaluated the expression of PCNA in lichen planus, keratosis and normal oral mucosa. PCNA expression were higher in all layers of lichen planus than in both keratosis and normal oral mucosa, it indicates that the keratinocyte proliferation index is higher in lichen planus than in keratosis and normal mucosa.[15] These results were in accordance with our study.
In the present study, we assessed PCNA expression in a different layer of epithelium in different forms of OLP (basal, intermediate and superficial layers). It was found that erosive type showed higher expression of PCNA in all the layers followed by reticular and plaque type. Positive cell for PCNA in the superficial layer of epithelium suggests possible alterations in cell differentiation mechanisms, step essential to the malignant transformation of epithelium.[5]
Conclusion
PCNA is a good marker to assess proliferation status of lesions. Its expression is associated with DNA repair process, initiation, promotion and progression of cancer. Out of three types of lichen planus, erosive type showed greater PCNA expression, followed by reticular and least in plaque type. This correlates with its greater proliferative ratio and increased chances of malignant transformation. This study thus emphasizes the need for timely management and long-term follow-up with erosive type when compared with reticular and plaque type.
Regarding the PCNA expression in different cell layers, the positive cell for PCNA in the superficial layer of epithelium suggests possible alterations in cell differentiation mechanisms, a step essential to the malignant transformation of epithelium. In the future, more studies are required to determine the significance of PCNA expression in various layers.
Acknowledgments
My heartful gratitude to Dr. Preeti Nayak, MDS, Reader, A J Institute of Dental Sciences, Mangalore and Dr. Nupura Vibhute, MDS, Reader, Department of Oral Pathology and Microbiology, School of Dental Sciences, Krishna Institute of Medical Sciences University, Karad.
Source of Support
Nil.
Conflict of Interest
None declared.
References
- Sousa FA, Rosa LE. Oral lichen planus: Clinical and histopathological considerations. Braz J Otorhinolaryngol 2008;74:284-92.
- Scully C, el-Kom M. Lichen planus: Review and update on pathogenesis. J Oral Pathol 1985;14:431-58.
- Edwards PC, Kelsch R. Oral lichen planus: Clinical presentation and management. J Can Dent Assoc 2002;68:494-9.
- Laeijendecker R, Van Joost T, Tank B, Oranje AP, Neumann HA. Oral lichen planus in childhood. Pediatr Dermatol 2005;22:299-304.
- de Sousa FA, Paradella TC, Carvalho YR, Rosa LE. Comparative analysis of cell proliferation ratio in oral lichen planus, epithelial dysplasia and oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal 2009;14:e563-7.
- Sousa FA, Paradella TC, Carvalho YR, Rosa LE. Immunohistochemical expression of PCNA, p53, bax and bcl-2 in oral lichen planus and epithelial dysplasia. J Oral Sci 2009;51:117-21.
- Burkhart NW, Burker EJ, Burkes EJ, Wolfe L. Assessing the characteristics of patients with oral lichen planus. J Am DentAssoc 1996;127:648,651-2,655.
- Jungell P. Oral lichen planus. A review. Int J Oral Maxillofac Surg 1991;20:129-35.
- Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L, Bucci E. Immune activation and chronic inflammation as the cause of malignancy in oral lichen planus: Is there any evidence? Oral Oncol 2004;40:120-30.
- Lee JJ, Kuo MY, Cheng SJ, Chiang CP, Jeng JH, Chang HH, et al. Higher expressions of p53 and proliferating cell nuclearantigen (PCNA) in atrophic oral lichen planus and patients with areca quid chewing. Oral Surg Oral Med Oral PatholOral Radiol Endod 2005;99:471-8.
- Jungell P. Oral lichen planus. A review. Int J Oral Maxillofac15. da Silva Fonseca LM, do Carmo MA. Identification of theSurg 1991;20:129-35.
- Mollaoglu N. Oral lichen planus: A review. Br J Oral Maxillofac Surg 2000;38:370-7.
- Eisenberg E. Oral lichen planus: A benign lesion. J OralMaxillofac Surg 2000;58:1278-85.
- Mitamura J. Onodera K, Ooya K. Histopathological and immunohistochemical study of oral lichen planus in the buccal mucosa: Relationship between clinicopathological features and histometrical analysis. Oral Med Pathol 2008:13;1-6.
- Xue JL, Fan MW, Wang SZ, Chen XM, Li Y, Wang L. A clinical AgNORs, PCNA and ck16 proteins in oral lichen planus lesions. Oral Dis 2001;7:344-8.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.