Impact of Family Background of Cardio-Metabolic Disorders and Associated Risk Factors on Total Adiponectin in Children
2 Department of Chemical Pathology, Nnamdi Azikiwe University Awka, Anambra, Nigeria, Email: okphenchi@gmail.com
Citation: Ulasi T, et al. Impact of Family Background of Cardio-Metabolic Disorders and Associated Risk Factors on Total Adiponectin in Children. Ann Med Health Sci Res. 2021;11:1411-1417.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Adiponectin is a novel hormone with unique biologic functions. Objective: Evaluating adiponectinpattern among children with a family history of cardio-metabolic diseases and risk factors may shed insight into the pathophysiologic mechanism behind these diseases among those at risk. Methods: 101 healthy children, aged 4 to 12 years, were enrolled in this study. Family history of diabetes, hypertension, obesity in both first and second degree relatives were established using Intervieweradministered questionnaires to the study participants’ caregivers. Other data collated include the smoking history, occupation and highest educational attainment of the caregivers and birth weight of the study participants. Venous blood samples were assayed foradiponectin using ELISA kits. Data were analyzed using SPSS software version 21 with the significance level for tests of associations done set at p<0.05. Results: Of 101 children, 53 subjects were males (52.5%) with a male to female ratio of 0.9:1. The mean age of all subjects was 7.8 ± 2.3 years. Children with a positive family history of type 2 diabetes mellitus, hypertension and obesity had higher mean adiponectin values than those without a family history, but these differences were not statistically significant. A negative trend was observed among subjects with a positive history of smoking and increased birth weight (p>0.05). No association was detected between adiponectin level and socioeconomic class. Conclusion: Future related studies conducted among subjects of African ancestry should explore the role of adiponectin resistance in the progression of cardio-metabolic diseases.
Keywords
Adiponectin; Children; Familial; Off-spring; African; Cardio-metabolic disorders; Obesity; Body mass index; Type 2 diabetes mellitus; Hypertension; Cardiovascular disorders; Atherosclerosis; Lipid disorders; Adipokine; Nigeria; Endocrinology
Introduction
Adiponectin gene is located at chromosome 3q27 of the human gene. [1] The translation of this gene yields a 244 amino-acid adipose-tissue protein named adiponectin which is recognized as the most abundant adipokine in the human body. Since its discovery, three isoforms have been isolated, namely Low Molecular Weight (LMW) trimers, Medium Molecular Weight (MMW) hexamers, and High Molecular Weight oligomers (HMW). [2] Together, these isoforms form total adiponectin with HMW oligomers proven to have the highest biologic potency. [2,3]
Adiponectin greatly enhances insulin sensitivity in target tissues. [4] It also has both anti-inflammatory and anti-oxidative properties, boosting a favorable lipid profile. [5] These biologic functions play an essential role in modulating certain chronic noncommunicable diseases whose pathophysiologic mechanisms are in direct contradistinction to adiponectin functions. Some of these diseases include type 2 diabetes mellitus, cardiovascular diseases, and obesity-related illnesses, with most comparative researches demonstrating an inverse relationship between the aforementioned diseases and blood adiponectin levels. [6-9] Several factors can regulate blood adiponectin levels. Higher adiponectin concentrations are observed in young children of prepubertal age. [10] Similarly, female gender and normal body mass indexes are associated with an increased adiponectin blood level. [11,12] Ethnicity also plays a crucial role, further demonstrating the impact of varied individual genomes on blood adiponectin level and function. [13,14] Notably, several studies have shown adiponectin heritability to account for 30%-93% of blood pattern. [15-19] In one of such studies conducted among African-Americans, adiponectin heritability pattern accounted for as high as 82% of its blood variability. [18] However, this study was confounded by mixed ancestry of European descent. A more recent study carried out among Afro-Caribbean populations in Tobago and primarily of West African descent reported a 33.2% influence on blood distribution. [20] Since lower adiponectin values is a risk factor of Type 2 diabetes mellitus, cardiometabolic disorders and obesity-related illnesses, the above findings on adiponectin heritability on its blood distribution do shed a profound insight behind the increased risk of these diseases among offspring with a positive family history.
Thus, understanding the impact of a family history of these diseases on the blood adiponectin distribution of our study participants in apparent healthy state can add to the growing body of knowledge that seeks to comprehend adiponectin role in the evolution of associated metabolic diseases.
Materials and Methods
This study was carried out in Nnewi, a cosmopolitan town in Anambra State, Nigeria, where most of its residents are of Igbo ethnicity. 101 apparently healthy children aged 4 to 12 years were randomly recruited from primary schools in Nnewi to participate in this study. The researcher interviewed the caregivers of the study participants to establish a family history of Type 2 diabetes mellitus, hypertension, and obesity in both first and second-degree relatives. We also obtained a history of passive and active smoking within the subjects’ residential area and birth weights [Figure 1]. The socioeconomic class was ascertained based on the highest educational attainment and occupation of both parents as described by Oyedeji. [21] Ethical approval from an independent Ethics committee was obtained prior to the start of this study. In addition, informed consent and assent were obtained from the caregivers of the study participants and subjects above six years respectively.
Blood sample collection and adiponectinassay
Five milliliters (5 ml) of venous whole blood was collected from each subject from 7 am to 9 am following an 8 hour overnight fast. The blood was stored in labelled plain sample bottles and spun in a centrifuge for 10 minutes at 3000 revolutions per minute. Two milliliters of the supernatant serum were harvested and stored at-25°C for a month before a two-batch analysis was conducted to avoid loss of bioactivity and contamination of the samples. [22] Total adiponectin was measured using Elabscience Human ADP/Acrp30 (Adiponectin) ELISA kits from Guangdong Science and Technology Industry Park, Wuhan, Republic of China; Catalog No.: E-EL-H0004). The kit’s minimum detectable dose was 0.47 ng/ml with a detection range of 0.78-50 ng/ml. The coefficient of variation was <10%.
Statistical Analysis
All statistical analysis was performed using the Statistical Software Package SPSS version 22 (SPSS Inc. Chicago IL, USA). Categorical data were expressed as frequencies and percentages, while continuous data were presented as mean and standard deviations. Descriptive statistics were employed to summarize all socio-demographic characteristics of study participants. For comparison of means of parametric quantitative variables between two groups, student t-test was utilized while one-way analysis of variance (ANOVA) was applied in more than two groups. Data visualizations were obtained with the ggplot2 package in R programming version 3.6.3.[23]
Results
A total of 101 primary school children aged 4 to 12 years were randomly recruited for this study. Of this, 53 (52.5%) were males with a male to female ratio of 0.9:1. The mean age of all subjects was 7.8 ± 2.3 years with no significant difference in age across gender. In all, the body mass indexes of all subjects that participated in this study were within the normal range, with no significant difference observed across both genders. The mean total adiponectin level was 4.51 ± 2.08 g/ml [Table 1].
| All | Girls | Boys | p | |
|---|---|---|---|---|
| No of Subjects (%) | 101 (100) | 48 (47.5) | 53 (52.5) | 0.287 |
| Age (years) | 7.8(2.3) | 7.57(2.29) | 8.15(2.29) | 0.207 |
| Weight (kg) | 26.06(6.64) | 25.14(6.09) | 27.07(7.13) | 0.144 |
| Height (cm) | 127.91(12.99) | 126.02(12.33) | 126.02(12.33) | 0.125 |
| BMI (kgm-2) | 15.64(1.31) | 15.59(1.18) | 15.70(1.44) | 0.666 |
| Birth weight (kg) | 3.23 (0.63) | 3.22 (0.67) | 3.23 (0.60) | 0.941 |
| Adiponectin (ng/ml) | 4.51(2.08) | 4.59(2.31) | 4.45(1.86) | 0.729 |
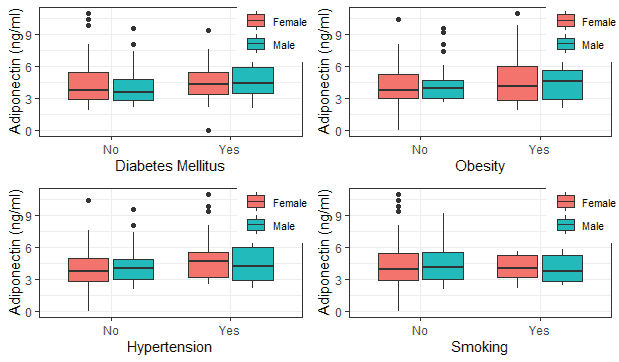
In Table 2, most of the caregivers reported no history of smoking, whereas the distribution of other reported disease conditions was essentially the same. Higher adiponectin values were observed among subjects with a positive family history of Type 2 diabetes mellitus, hypertension and obesity, but this was not significant. In contrast, those with a positive history of smoking had lower adiponectin values [Tables 3 and 4]. No obvious pattern was observed among subjects of different social classes [Table 5].
| Cardio-metabolic diseases and risk factors | No history (%) | Positive history (%) | |
|---|---|---|---|
| All subjects | Hypertension | 49(48.5) | 52(51.5) |
| Obesity | 57(56.4) | 44(43.6) | |
| Type 2 Diabetes | 55(54.5) | 46(45.5) | |
| Smoking | 84(83.2) | 17(16.8) | |
| Males | Hypertension | 27(50.9) | 26(49.1) |
| Obesity | 30(56.6) | 23(43.4) | |
| Type 2 Diabetes | 30(56.6) | 23(43.4) | |
| Smoking | 44(83.0) | 9(17.0) | |
| Females | Hypertension | 22(45.8) | 26(54.2) |
| Obesity | 27(56.3) | 21(43.8) | |
| Type 2 Diabetes | 25(52.1) | 23(47.9) | |
| Smoking | 40(83.3) | 8(16.7) |
Table 2: Family history of associated cardio-metabolic disorders and risk factors.
| Cardio-metabolic diseases and risk factors | No history (%) | Positive history (%) | p value | |
|---|---|---|---|---|
| All Subjects | Hypertension | 4.19(1.95) | 4.82(2.16) | 0.128 |
| Obesity | 4.42(2.00) | 4.63(2.19) | 0.612 | |
| Type 2 Diabetes | 4.44(2.25) | 4.60(1.86) | 0.705 | |
| Smoking | 4.57(2.13) | 4.26(1.83) | 0.581 | |
| Males | Hypertension | 4.30(1.81) | 4.59(1.93) | 0.576 |
| Obesity | 4.46(2.00) | 4.43(1.70) | 0.948 | |
| Type 2 Diabetes | 4.21(1.92) | 4.75(1.77) | 0.296 | |
| Smoking | 4.46(1.79) | 4.40(2.28) | 0.937 | |
| Females | Hypertension | 4.05(2.15) | 5.05(2.38) | 0.139 |
| Obesity | 4.38(2.04) | 4.86(2.65) | 0.478 | |
| Type 2 Diabetes | 4.72(2.62) | 4.45(1.98) | 0.688 | |
| Smoking | 4.69(2.47) | 4.10(1.29) | 0.518 |
Table 3: Distribution of adiponectin (ng/ml) based on family history of cardio-metabolic disorders and risk factors.
| Social Class | All Subjects (%) | Male (%) | Female (%) |
|---|---|---|---|
| Upper | 38(37.6) | 16(30.2) | 22(45.8) |
| Middle | 43(42.6) | 22(41.5) | 21(43.8) |
| Lower | 20(19.8) | 15(28.3) | 5(10.4) |
| Total | 101(100) | 53(100) | 48(100) |
Table 4: Distribution of socioeconomic classes of the subjects.
| Social Class | All | p value | Male (SD) | p value | Female (SD) | p value |
|---|---|---|---|---|---|---|
| Subjects (SD) | ||||||
| Upper | 4.83(2.21) | 4.79(2.07) | 4.86(2.35) | |||
| Middle | 4.24(2.06) | 0.444 | 4.01(1.59) | 0.351 | 4.49(2.47) | 0.658 |
| Lower | 4.50(1.85) | 4.72(1.97) | 3.84(1.43) |
Table 5: Adiponectin values across different socio-economic classes.
Tables 6 and 7 illustrated a higher trend in adiponectin values when the frequency of aggregated reports of a family history of cardio-metabolic diseases and smoking were greater, but this was not significant.
| Frequency | All Subjects (%) | Male (%) | Female (%) |
|---|---|---|---|
| 0 | 23(22.8) | 14(26.4) | 9(18.8) |
| 1 | 22(21.8) | 10(18.9) | 12(25.0) |
| 2 | 31(30.7) | 16(30.2) | 15(31.3) |
| 3 | 25(24.8) | 13(24.5) | 12(25.0) |
| Total | 101(100) | 53(100) | 48(100) |
Table 6: Frequency of aggregated family history of Type 2 diabetes mellitus, hypertension, obesity and smoking among the study participants.
| Frequency | All Subjects (SD) | p | Male (SD) | p | Female (SD) | p |
|---|---|---|---|---|---|---|
| 0 | 4.32(1.91) | 4.24(1.61) | 4.44(2.41) | |||
| 1 | 4.41(2.32) | 0.768 | 4.78(2.39) | 0.411 | 4.10(2.32) | 0.817 |
| 2 | 4.43(2.33) | 3.94(1.94) | 4.94(2.67) | |||
| 3 | 4.90(1.7) | 5.03(1.52) | 4.75(1.94) |
Table 7: Distribution of adiponectin based on frequency of aggregated family history of Type 2 diabetes mellitus, hypertension, obesity and smoking among the study participants.
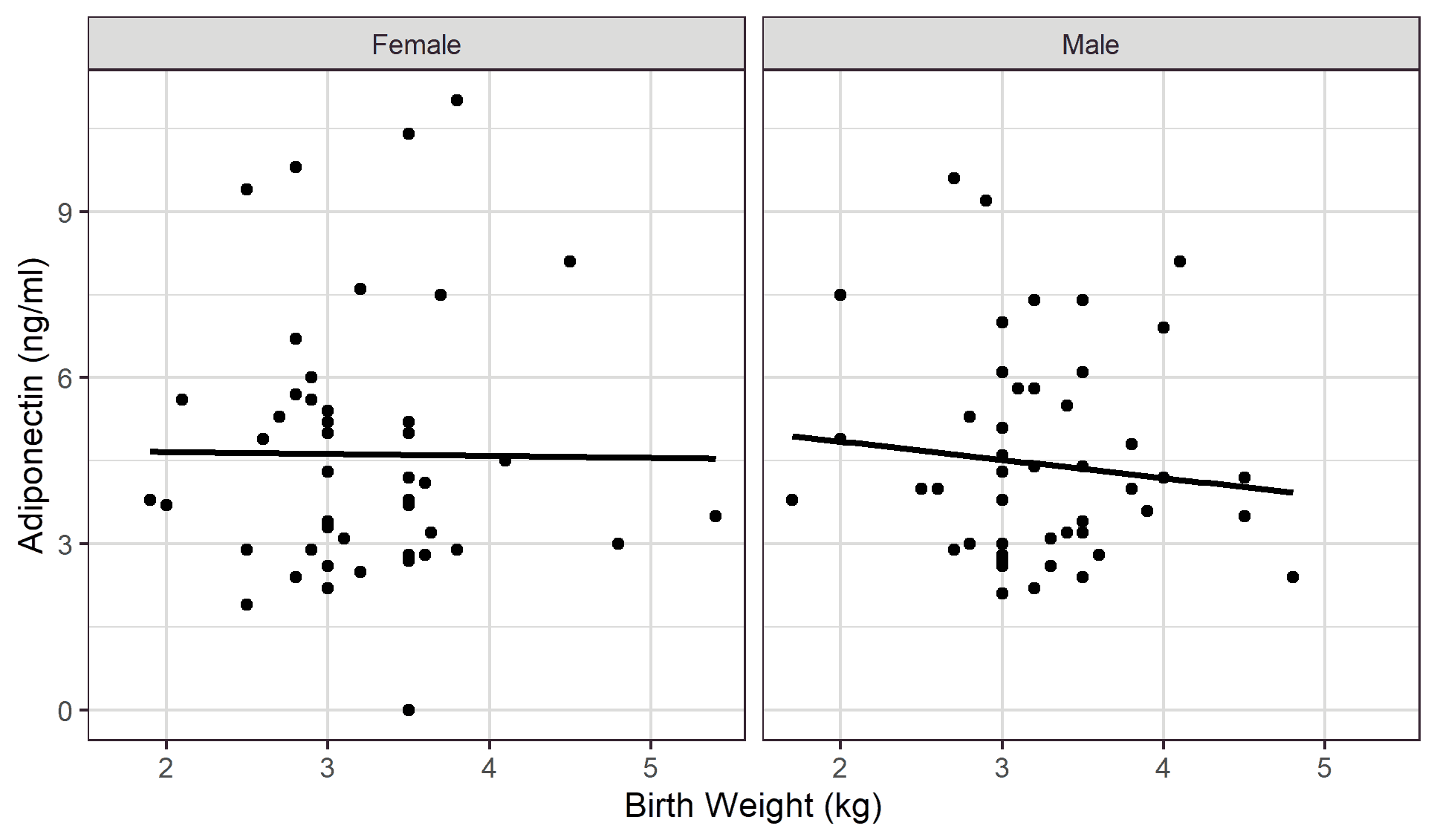
An inverse relationship was demonstrated between the subjects’ birth weight and total adiponectin concentrations in both male and female children with correlation values of -0.106; p=0.466 and -0.010; p=0.948 respectively [Figure 2].
Discussion
Although many studies have established a significant influence of adiponectin heritability on blood variability, fewer studies have shown the impact of a family history of associated diseases and the risk factors on the adiponectin profile of the progeny. [24-27] To the best of our knowledge, this research is the first to address this research topic among Nigerian children. Elimination of known confounders, namely age, sex, puberty and increased body mass, was addressed through the random selection of prepubertal age subjects with normal body mass compositions. Adiponectin remains an area of global research interest due to its unique biological functions that possess the vast potential to unlock the nuances behind the pathophysiologic mechanisms and targeted therapy of non-communicable diseases of key public interest. [28,29]
Subjects with a family background of type 2 diabetes mellitus, hypertension, and obesity demonstrated higher mean adiponectin concentrations than their peers who did not share a similar history. Although this was not significant, we considered this paradoxical finding to be intriguing and at variance with other published works. [24–27] An inverse relationship exists between adiponectin and markers of insulin resistance in Type 2 diabetes mellitus and obesity, pro-inflammatory mediators in obesity and atherosclerosis in hypertension. [30-35] Notwithstanding that adiponectin assay was not conducted for other family members with a background the diseases of interest, it stands to reason that a lower adiponectin level should be expected. Therefore, in keeping with adiponectin heritability pattern, which should account for a significant degree of adiponectin distribution in offsprings, a mean lower adiponectin was expected among subjects with a positive family history of type 2 diabetes mellitus, hypertension and obesity, thus providing some rare insight to the transmissibility of these diseases. Finally, lower adiponectin values can serve as a biomarker predicting an increased risk of cardio-metabolic disorders over time among children of apparent good health. [35,36] Hence, the discovery of a paradoxical outcome supports the possibility of an inherent adiponectin resistant state. In severe insulin resistance, receptor insensitivity to adiponectin can lead to hyperadiponectinaemia, which serves as an initial compensatory mechanism; however, persistence of this phenomenon may ultimately desensitiseadiponectinproducing adipocytes leading to lower blood adiponectin values. [23,37-39] Insulin resistance can occur in children with a positive family history of type 2 diabetes mellitus. [25,40] An alternative explanation to the paradoxical finding obtained may be attributed to a re-distribution in the quantitative values of the three isomeric forms of adiponectin in favor of the isomers with lesser biologic activity namely the trimer and hexamers, leading to a resistant state which can trigger an increase in hormone production. [41,42]
In this study, birth weight negatively correlated with adiponectin across both genders, but this was statistically insignificant. This observation is similar to the findings reported by Fonseca et al. [43] but is dissimilar to past studies, which demonstrated varied reports on the association of serum adiponectin levels and birth weights. [44-47] Studies that observed a significant positive correlation with increasing birth weight adduced that subjects with low birth weights have a greater risk of developing type 2 diabetes and cardiovascular diseases later on in adulthood than those with normal birth weights. [45,46,48] A similar explanation was proposed in studies that found a significant negative correlation with increasing birth weight. [44,47] It was speculated that large for gestational age birth weights could be associated with early obesity postnatally with a potential increase in cardiovascular and metabolic risk in adulthood. [49–51] The negative trend observed between birth weight and adiponectin in our study may also be related to the incidental discovery that the mean birth weight of subjects with a positive family history of Type 2 diabetes mellitus was significantly lower than those without a similar family history. Reasons for the higher birth weight among those with a family background of type 2 diabetes mellitus remains unknown.
Smoking, both active and passive, constitute a potential risk factor for cardio-metabolic illnesses. Smoking triggers the onset of atherosclerosis along the vasculature linings of exposed subjects. The present study found no statistically significant association between the family history of smoking and adiponectin levels. The observation is in agreement with the findings of Khanolkar and colleagues [52] from Sweden and Punthakee et al. [33] from Canada. It however differs from those of Papaioannou and co-workers [53] from Greece, who demonstrated a significant decrease in adiponectin level of children with a positive family history of smoking. Several other studies including an expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents, have consistently demonstrated the adverse effect of cigarette smoking, whether passive or active. [54-57] Also, an experimental animal study illustrated that smoking inhibits adiponectin secretion by reducing mRNA expression. [58] The lack of significance in this study may be explained by the lower proportion of subjects with a positive smoking history. This limitation can confer statistically a reduced power to detect any difference if any.
Past research has demonstrated a paucity of evidence concerning the relationship between adiponectin levels and socioeconomic status. We found no association and this corroborates with those of Lausten-Thomsen and colleagues [59] and Khanolkar et al. [52], who both reported no significant difference in adiponectin across different socioeconomic classes. In contrast, Buchan et al. [30] and Davis and co-workers [60] observed significantly lower mean adiponectin values among subjects from the lower class than those from the upper class. However, it should be noted that these studies had utilized different methodological approaches in classifying social classes at variance to that used in this study, which may explain the conflicting results. Several published works have observed an increased incidence of noncommunicable diseases such as hypertension, obesity, metabolic syndrome and type 2 diabetes mellitus and their risk factors among subjects from the lower class. [61-63] Cited reasons include reduced physical activity, poor dietary patterns, smoking and alcohol consumption. [63]
The validity of the self-reported family history of evaluated diseases and the subjects’ birth weight could not be verified through existing health care records. In Nigeria, the prevalent culture of poor record-keeping and paper records poses a significant undertaking in confirming the validity of the reported histories among family members. Notwithstanding, this limitation does not invalidate completely the reports given as the rich social culture inherent in our society ensures that family members are mostly aware of their closest family unit’s health conditions. More so, most of the caregivers in this study are educated as shown by the distribution of social classes, thus can recall their wards’ birth weight values and understand the concept of the diseases of interest.
Conclusion
More exploratory studies are needed to investigate adiponectin distribution in subclinical states, particularly among children at risk. A key area of interests as it concerns our unique ethnicity should include adiponectin resistance and the distribution of its various isoforms.
Competing Interests
The authors declare that they have no competing interests.
REFERENCES
- Pollin TI, Tanner K, O’Connell JR, Ott SH, Damcott CM, Shuldiner AR, et al. Linkage of plasma adiponectin levels to 3q27 explained by association with variation in the APM1 gene. Diabetes. 2005;54:268-274.
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746-26749.
- Araki S, Dobashi K, Kubo K, Asayama K, Shirahata A. High molecular weight, rather than total, adiponectin levels better reflect metabolic abnormalities associated with childhood obesity. J Clin Endocrinol Metab. 2006;91:5113-5116.
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941-946.
- Magge SN, Stettler N, Koren D, Katz LEL, Gallagher PR, Mohler ER, et al. Adiponectin is associated with favorable lipoprotein profile, independent of BMI and insulin resistance, in adolescents. J Clin Endocrinol Metab. 2011;96:1549-1554.
- Atwa H, Mesbah B, Sad A. Adiponectin could be a comprehensive marker of metabolic syndrome in obese children. South African J Clin Nutr. 2012;25:53-56.
- Pyrzak B, Ruminska M, Popko K, Demkow U. Adiponectin as a biomarker of the metabolic syndrome in children and adolescents. Eur J Med Res. 2010;15:147-151.
- Cambuli VM, Musiu MC, Incani M, Paderi M, Serpe R, Marras V, et al. Assessment of adiponectin and leptinas biomarkers of positive metabolic outcomes after lifestyle intervention in overweight and obese children. J Clin Endocrinol Metab. 2008;93:3051-3057.
- Klünder-Klünder M, Flores-Huerta S, García-Macedo R, Peralta-Romero J, Cruz M. Adiponectin in eutrophic and obese children as a biomarker to predict metabolic syndrome and each of its components. BMC Public Health. 2013;13:88.
- Böttner A, Kratzsch J, Müller G, Kapellen TM, Blüher S, Keller E, et al. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053-4061.
- Tsou P, Jiang Y, Chang C, Wei J, Sung F, Lin C, et al. Sex-related differences between adiponectin and insulin resistance in schoolchildren. Diabetes Care. 2004;27:308-313.
- Reinehr T, Roth C, Menke T, Andler W. Adiponectin before and after weight loss in obese children. J Clin Endocrinol Metab. 2004;89:3790-3794.
- Yamauchi DM, Dilts JR, Bovenkerk JE,Saha C, Pratt JH, Considine RV. Lower serum adiponectin levels in African-American boys. Obes Res. 2003;11:1384-1390.
- Bush NC, Darnell BE, Oster RA, Goran MI, Gower BA. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes. 2005;54:2772-2778.
- Chuang LM, Chiu YF, Sheu WHH, Hung YJ, Ho LT, Grove J, et al. Biethnic comparisons of autosomal genomic scan for loci linked to plasma adiponectin in populations of Chinese and Japanese origin. J Clin Endocrinol Metab. 2004;89:5772-5778.
- Lindsay RS, Funahashi T, Krakoff J, Matsuzawa Y, Tanaka S, Kobes S, et al. Genome-wide linkage analysis of serum adiponectin in the Pima Indian population. Diabetes. 2003;52:2419-2425.
- Menzaghi C, Ercolino T, Salvemini L, Coco A, Kim SH, Fini G, et al. Multigenic control of serum adiponectin levels: Evidence for a role of the APM1 gene and a locus on 14q13. Physiol Genomics. 2005;19:170-174.
- Guo X, Saad MF, Langefeld CD, Williams AH, Cui J, Taylor KD, et al. Genome-wide linkage of plasma adiponectin reveals a major locus on chromosome 3q distinct from the adiponectin structural gene: The IRAS Family Study. Diabetes. 2006;55:1723-1730.
- Butte NF, Comuzzie AG, Cai G, Cole SA, Mehta NR, Bacino CA. Genetic and environmental factors influencing fasting serum adiponectin in hispanic children. J Clin Endocrinol Metab. 2005;90:4170-4176.
- Miljkovic-Gacic I, Wang X, Kammerer CM, Bunker CH, Wheeler VW, Patrick AL, et al. Genetic determination of adiponectin and its relationship with body fat topography in multigenerational families of African heritage. Metabolism. 2007;56:234-238.
- Oyedeji G. Socioeconomic and cultural background of hospitalized children in Ilesa, Nigeria. Niger J Paediatr. 1985;12:111-117.
- ELISA Kits & amp; Reagents for Test & amp; Assay, inquiry price on Elabscience.com. 2018.
- Lin HV, Kim JY, Pocai A, Rossetti L, Shapiro L, Scherer PE, et al. Adiponectin resistance exacerbates insulin resistance in insulin receptor transgenic/knockout mice. Diabetes. 2007;56:1969-1976.
- Oh YJ, Nam HK, Rhie YJ, Park SH, Lee KH. Low serum adiponectin levels in Korean children with a family history of type 2 diabetes mellitus. Horm Res pædiatrics. 2012;77:382-387.
- Pellmé F, Smith U, Funahashi T, Matsuzawa Y, Brekke H, Wiklund O, et al. Circulating adiponectin levels are reduced in nonobese but insulin-resistant first-degree relatives of type 2 diabetic patients. Diabetes. 2003;52:1182-1186.
- Furuhashi M, Ura N, Higashiura K, Miyazaki Y, Murakami H, Hyakukoku M, et al. Low adiponectin level in young normotensive men with a family history of essential hypertension. Hypertens Res. 2005;28:141-146.
- Nasser M, Omar S, Mohammed A, Khalid A, Hussein A. Adipocytokines as influenced by family history of type 2 diabetes and smoking in non-diabetic subjects. Clin Med Insights Endocrinol Diabetes. 2008;1:13-19.
- Nawrocki AR SP. Keynote review: The adipocyte as a drug discovery target. Drug Discov Today 2005;10:1219-1230.
- Lim S, Quon MJ, Koh KK. Modulation of adiponectin as a potential therapeutic strategy. Atherosclerosis. 2014;233:721-728.
- Wannamethee SG, Tchernova J, Whincup P, Lowe GD, Rumley A, Brown K, et al. Associations of adiponectin with metabolic and vascular risk parameters in the British Regional Heart Study reveal stronger links to insulin resistance-related than to coronory heart disease risk-related parameters. Int J Obes (Lond). 2007;31:1089-1098.
- Medina-Bravo P, Meza-Santibáñez R, Rosas-Fernández P, Galván-Duarte R, Saucedo-García R, Velázquez-López L, et al. Decrease in serum adiponectin levels associated with visceral fat accumulation independent of pubertal stage in children and adolescents. Arch Med Res. 2011;42:115-121.
- Gil-Campos M, Cañete RR, Gil A. Adiponectin, the missing link in insulin resistance and obesity. Clin Nutr. 2004;23:963-974.
- Punthakee Z, Delvin EE, O’Loughlin J, Paradis G, Levy E, Platt RW, et al. Adiponectin, adiposity, and insulin resistance in children and adolescents. J Clin Endocrinol Metab. 2006;91:2119-2125.
- Hug C, Lodish HF. The role of the adipocyte hormone adiponectin in cardiovascular disease. Curr Opin Pharmacol. 2005;5:129-134.
- Lu JY, Huang KC, Chang LC, Huang YS, Chi YC, Su TC, et al. Adiponectin: A biomarker of obesity-induced insulin resistance in adipose tissue and beyond. J Biomed Sci 2008;15:565-576.
- Shaibi GQ, Cruz ML, Weigensberg MJ, Toledo-Corral CM, Lane CJ, Kelly LA, et al. Adiponectin independently predicts metabolic syndrome in overweight Latino youth. J ClinEndocrinolMetab. 2007;92:1809-1813.
- Semple RK, Soos MA, Luan J, Mitchell CS, Wilson JC, Gurnell M, et al. Elevated plasma adiponectin in humans with genetically defective insulin receptors. J Clin Endocrinol Metab. 2006;91:3219-3223.
- Semple RK, Halberg NH, Burling K, Soos MA, Schraw T, Luan J, et al. Paradoxical elevation of high-molecular weight adiponectin in acquired extreme insulin resistance due to insulin receptor antibodies. Diabetes. 2007;56:1712-1717.
- Mullen KL, Pritchard J, Ritchie I, Snook LA, Chabowski A, Bonen A, et al. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol - RegulIntegr Comp Physiol. 2009;296.
- Arslanian SA, Bacha F, Saad R, Gungor N. Family history of type 2 diabetes is associated with decreased insulin sensitivity and an impaired balance between insulin sensitivity and insulin secretion in white youth. Diabetes Care. 2005;28:115-119.
- Kaser S, Tatarczyk T, Stadlmayr A, Ciardi C, Ress C, Tschoner A, et al. Effect of obesity and insulin sensitivity on adiponectin isoform distribution. Eur J Clin Invest. 2008;38:827-834.
- Retnakaran R, Hanley AJG, Connelly PW, Maguire G, Sermer M, Zinman B. Low serum levels of high-molecular weight adiponectin in Indo-Asian women during pregnancy: Evidence of ethnic variation in adiponectin isoform distribution. Diabetes Care. 2006;29:1377-1379.
- Fonseca MJ, Santos AC. Umbilical cord blood adipokines and newborn weight change. Arch Gynecol Obstet. 2015;291:1037-1040.
- Mohamed S, El-Askary A, Megahed A. Adiponectin and resistin levels in umbilical serum of term neonates and relation to birth weight. Neonatal Pediatr Med. 2017;03:1-6.
- Cianfarani S, Martinez C, Maiorana A, Scire G, Spadoni GL, Boemi S. Adiponectin levels are reduced in children born small for gestational age and are inversely related to postnatal catch-up growth. J Clin Endocrinol Metab. 2004;89:1346-1351.
- Crume TL, Scherzinger A, Stamm E, McDuffie R, Bischoff KJ, Hamman RF, et al. The long-term impact of intrauterine growth restriction in a diverse U.S. cohort of children: the EPOCH study. Obesity (Silver Spring). 201;22:608-615.
- Sancakli O, Darendeliler F, Bas F, Gokcay G, Disci R, Aki S, et al. Insulin, adiponectin, IGFBP-1 levels and body composition in small for gestational age born non-obese children during prepubertal ages. ClinEndocrinol (Oxf). 2008;69:88-92.
- Tamakoshi K, Yatsuya H, Wada K, Matsushita K, Otsuka R, Sugiura K, et al. Low birth weight is associated with reduced adiponectin concentration in adult. Ann Epidemiol. 2006;16:669-674.
- Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011. Developmental overnutrition—an old hypothesis with new importance? Int J Epidemiol. 2013;42:7-29.
- Fraser A, Lawlor DA. Long-term health outcomes in offspring born to women with diabetes in pregnancy. Curr Diab Rep. 2014;14:489.
- Chiavaroli V, Marcovecchio ML, de Giorgis T, Diesse L, Chiarelli F, Mohn A. Progression of cardio-metabolic risk factors in subjects born small and large for gestational age. PLoS One. 2014;9:e104278.
- Khanolkar AR, Byberg L, Koupil I. Parental influences on cardiovascular risk factors in Swedish children aged 5–14 years. Eur J Public Health. 2012;22:840-847.
- Papaioannou HI, Stakos DA, Tziakas DN, Chalikias G, Tsigalou C, Kartali S, et al. Plasma leptin and adiponectin concentrations in healthy, non-obese children. J Pediatr Endocrinol Metab. 2011;24:313–8.
- de Jesus JM. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics. 2011;128:S213-S256.
- Yun M, Li S, Sun D, Ge S, Lai C-C, Fernandez C, et al. Tobacco smoking strengthens the association of elevated blood pressure with arterial stiffness: the Bogalusa heart study. J Hypertens. 2015;33:266-274.
- Tsai J, Guo F, Chen S, Leu B, Chiu T, Chen C, et al. Smokers show reduced circulating adiponectin levels and adiponectin mRNA expression in peripheral blood mononuclear cells. Atherosclerosis 2011;218:168-173.
- Pardo I, Geloneze B, Tambascia M, Barros A. Inverse relationship between cord blood adiponectin concentrations and the number of cigarettes smoked during pregnancy. Diabetes, Obes Metab. 2005;7:144-147.
- Iwashima Y, Katsuya T, Ishikawa K, Kida I, Ohishi M, Horio T, et al. Association of hypoadiponectinemia with smoking habit in men. Hypertension. 2005;45:1094-1100.
- Lausten-Thomsen U, Christiansen M, Fonvig CE, Trier C, Pedersen O, Hansen T, et al. Reference values for serum total adiponectin in healthy non-obese children and adolescents. Clin Chim Acta. 2015;450:11-14.
- Davis S, Gebreab S, Xu R, Riestra P, Khan R, Sumner A, et al. Association of adiponectin with type 2 diabetes and hypertension in African American men and women: the Jackson Heart Study. Am Hear Assoc 2014;15:13.
- Grotto I, Huerta M, Sharabi Y. Hypertension and socioeconomic status. Curr Opin Cardiol. 2008;23:335-339.
- Rawshani A, Svensson A-M, Rosengren A, Eliasson B, Gudbjörnsdottir S. Impact of socioeconomic status on cardiovascular disease and mortality in 24,947 individuals with type 1 diabetes. Diabetes Care. 2015;38:1518-1527.
- Psaltopoulou T, Hatzis G, Papageorgiou N, Androulakis E, Briasoulis A, Tousoulis D. Socioeconomic status and risk factors for cardiovascular disease: Impact of dietary mediators. Hell J Cardiol. 2017;58:32-42.
Select your language of interest to view the total content in your interested language
Awards Nomination
20+ Million Readerbase
Google Scholar citation report
Citations : 24805
Annals of Medical and Health Sciences Research received 24805 citations as per google scholar report
Annals of Medical and Health Sciences Research peer review process verified at publons
Indexed in
PubMed Central Index Copernicus Emerging Sources Citation Index
Abstracted/Indexed in
- Include Baidu Scholar
- CNKI (China National Knowledge Infrastructure)
- EBSCO Publishing's Electronic Databases
- Exlibris – Primo Central
- Google Scholar
- Hinari
- Infotrieve
- National Science Library
- ProQuest
- TdNet
- African Index Medicus
 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.