Incidence of Malignancies Spondyloarthritis Patients Treated with Biologics vs Non-Biologics: Systematic Review and Meta-analysis
Citation: Alghamdi TA, et al. Incidence of Malignancies Spondyloarthritis Patients Treated with Biologics vs. Non-Biologics: A Systematic Review and Meta-analysis. Ann Med Health Sci Res. 2021;11: 1222-1227.
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Background: Biologic therapies have all demonstrated efficacy inside the treatment of AS. however, some patients do not reply to remedy (i.e., number one treatment failure) or enjoy diminished efficacy over the years (i.e., secondary treatment failure), while others experience side effects necessitating treatment discontinuation e.g. (incidence of malignancies). Aim: This work aims to assess the incidence of malignancies in spondyloarthritis (SpA) patients treated with biologics. Materials and Methods: A systematic search was performed over different medical databases to identify Internal Medicine studies, which studied the outcome of the Biologics group versus the Non-biologics group of spondyloarthritis (SpA) patients. Using the meta-analysis process, either with fixed or random-effects models, we conducted a meta-analysis on the overall incidence of malignancy as a primary outcome, and on the incidence of malignancy in TNF-A and IL-17 therapies (as secondary outcomes). Results: Eleven studies were identified involving 4395 patients, with 2878 patients in the Biologics group, and 1517 patients in the Non-biologics group. The meta-analysis process revealed a non-significant difference in the overall incidence of malignancy in the Biologics group compared to the Non-biologics group (p > 0.05), with also a non-significant difference in the incidence of malignancy in TNF-A and IL-17 users (p > 0.05). Conclusion: To conclude, we found no evidence for elevated risk of malignancy with the use of biologics in SpA. We were also unable to assess the long-term risk of biologics in SpA, which requires longer follow-up durations, and further investigatory studies.
Keywords
Malignancy; Spondyloarthritis; Biologics
Introduction
Spondyloarthritis (SpA) is a group of illnesses in large part associated with the main histocompatibility complex class I antigen HLA-B27. The SpA is classed into main subtypes, axial SpA (axSpA) and peripheral SpA. The term axSpA is used to explain patients with chronic back pain (normally inflammatory), alongside other clinical features of SpA, including arthritis, enthesitis, uveitis, dactylitis, inflammatory bowel diseases, and psoriasis. AxSpA is similarly subcategorized as ankylosing spondylitis (AS) or non-radiographic axSpA (nraxSpA). Patients with chronic back pain and features of SpA who do now not have radiographic evidence of sacroiliitis are classified as having nr-axSpA. Inflammatory diseases are often characterized by way of bone metabolism impairment: local and systemic bone loss in RA, destructive and efficient bone lesions in Spondyloarthritis (SpA). [1]
The principle aim of the SpA control is to reduce disease activity and control joint damage, improving the quality of life, and preserving the functional abilities of patients as well as autonomy and social participation. A success control of extraarticular clinical findings of SpA, which includes psoriasis, uveitis, and IBD, calls for energetic cooperation among the rheumatologist and other professionals, in collaboration with the primary care physician. Also, information and education of patients represent key factors for improving SpA outcome, in addition to rehabilitation represented via home exercises. [2]
TNF blockers had been the therapeutics of choice for almost 15 years in patients with axial spondyloarthritis (axSpA, inclusive of each radiographic axSpA, also known as AS, and non-radiographic axial SpA, nr-axSpA) if traditional treatment fails. Only currently capsules focused on IL-17A have also been shown to be effective for those diseases. For the past decade, the Th17 axis has been shown to play an important function within the pathogenesis of animal models of autoimmune sickness, indicating that it might also play a relevant function in the pathogenesis of immune-mediated sicknesses in human beings. Th17 cells produce IL-17 on stimulation with IL-23, however, cells from the innate immune machine also reply to IL-23 stimulation, probably not only by the secretion of IL-17. IL-23- independent production of IL-17 has additionally been reported. Moreover, it’s been shown the usage of tissue samples from AS patients that IL-17 seems to be more frequently produced by non-T cells than by T cells. [3]
TNF inhibitors, which includes infliximab, etanercept, adalimumab, golimumab, and certolizumab pegol, have all demonstrated efficacy inside the treatment of AS. However, some patients do not reply to remedy (i.e., number one treatment failure) or enjoy diminished efficacy over the years (i.e., secondary treatment failure), while others experience side effects necessitating treatment discontinuation e.g. (incidence of malignancies). [4]
This work aims to assess the incidence of malignancies in spondyloarthritis (SpA) patients treated with biologics.
Literature Review
Our review came following the (PRISMA) statement guidelines. [5]
Study eligibility
The included studies should be in English, a journal published article, and a human study describing spondyloarthritis (SpA) patients. The excluded studies were non-English or animal studies or describing other types of autoimmune patients (e.g. systemic lupus erythematosus “SLE”, or rheumatoid arthritis).
Study identification
Basic searching was done over the PubMed, Cochrane library, and Google scholar using the following keywords: Malignancy, Spondyloarthritis, Biologics.
Data extraction and synthesis
RCTs, clinical trials, and comparative studies, which studied the outcome of the Biologics group versus Non-biologics group of spondyloarthritis (SpA) patients, will be reviewed.
Outcome measures included overall incidence of malignancy as a primary outcome, and on the incidence of malignancy in TNF-A “tumor necrosis factor-alpha” and IL-17 “interleukin-17” therapies (as secondary outcomes).
Study selection
We found 270 records, 194 excluded based on title and abstract review; 76 articles are searched for eligibility by full-text review; 26 articles cannot be accessed; 23 studies were reviews and case reports; the desired drug not used in 16 studies leaving 11 studies that met all inclusion criteria.
Statistical analysis
After the pooling of data, Odds ratios (OR), with 95% confidence intervals (CI) were calculated, using MedCalc statistical software (Belgium). After the Q test of heterogeneity, the I2- statistics (either the fixed-effects model or the random-effects model) were done within the meta-analysis process.
Results
The included studies were published between 2009 and 2020.
Regarding patients’ characteristics, the total number of patients in all the included studies was 4395 patients, with 2878 patients in the Biologics group, and 1517 patients in the Non-biologics group, while their average follow-up time was (12 months) [Table 1]. [6-16]
| N | Author | Used biologic |
Number of patients | Minimum age |
Follow-up time (average months) |
||
|---|---|---|---|---|---|---|---|
| Total | Biologics group | Non-biologics group | |||||
| 1 | Dijkmans et al. [6] | TNF | 84 | 45 | 39 | -- | 24 |
| 2 | Kavanaugh et al. [7] | TNF | 405 | 292 | 113 | -- | 6 |
| 3 | Bao et al. [8] | TNF | 213 | 108 | 105 | 18 | 12 |
| 4 | Baeten et al. [9] | IL17 | 371 | 249 | 122 | 18 | 12 |
| 5 | McInnes et al. [10] | IL17 | 397 | 299 | 98 | 18 | 25 |
| 6 | P. J. Mease et al. [11] | IL17 | 606 | 404 | 202 | 18 | 12 |
| 7 | Kavanaugh et al. [12] | TNF | 100 | 51 | 49 | 18 | 6 |
| 8 | Nash et al. [13] | IL17 | 363 | 245 | 118 | 18 | 6 |
| 9 | Landewé et al. [14] | TNF | 305 | 152 | 153 | 18 | 17 |
| 10 | P. Mease et al. [15] | IL17 | 996 | 664 | 332 | 18 | 6 |
| 11 | Deodhar et al. [16] | IL17 | 555 | 369 | 186 | 18 | 5 |
| #Studies arranged via publication year. | |||||||
Table 1: Patients and study characteristics.
A meta-analysis study was done on 11 studies that described and compared the 2 different groups of patients; with an overall number of patients (N=4395) [Table 2]. [6-16]
| N | Author | Primary outcome | |
|---|---|---|---|
| The overall incidence of malignancy | |||
| Biologics group | Non-biologics group | ||
| 1 | Dijkmans et al. [6] | 4 | 0 |
| 2 | Kavanaugh et al. [7] | 3 | 0 |
| 3 | Bao et al. [8] | 1 | 0 |
| 4 | Baeten et al. [9] | 3 | 1 |
| 5 | McInnes et al. [10] | 3 | 0 |
| 6 | P. J. Mease et al. [11] | 3 | 1 |
| 7 | Kavanaugh et al. [12] | 0 | 3 |
| 8 | Nash et al. [13] | 2 | 0 |
| 9 | Landewé et al. [14] | 0 | 1 |
| 10 | P. Mease et al. [15] | 1 | 0 |
| 11 | Deodhar et al. [16] | 3 | 0 |
Table 2: Summary of outcome measures in all studies.
Each outcome was measured by:
Odds Ratio (OR)
• For the overall incidence of malignancy.
• For the incidence of malignancy in TNF-A users.
• For the incidence of malignancy in IL-17 users.
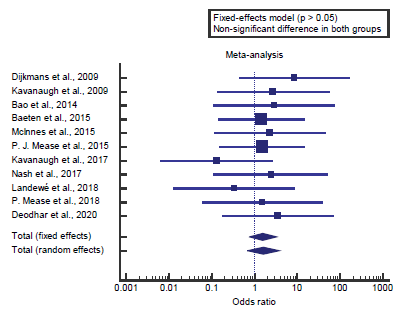
Concerning the primary outcome measure, we found 11 studies reported an overall incidence of malignancy with a total number of patients (N=4395). I2 (inconsistency) was 0% with a nonsignificant Q test for heterogeneity (p>0.05), so fixed-effects model was carried out; with overall OR= 1.58 (95% CI=0.757 to 3.309).
Using the fixed-effects model, the meta-analysis process revealed a non-significant difference in the overall incidence of malignancy in the Biologics group compared to the Nonbiologics group (p>0.05) [Figure 1].
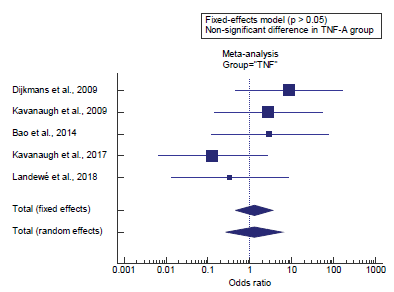
Concerning the secondary outcome measures, we found 5 studies reported the incidence of malignancy in TNF-A users. I2 (inconsistency) was 20.6% with a non-significant Q test for heterogeneity (p>0.05), so fixed-effects model was carried out; with overall OR= 1.26 (95% CI=0.458 to 3.502).
Using the fixed-effects model, the meta-analysis process revealed a non-significant difference in the incidence of malignancy in TNF-A users in the Biologics group compared to the Non-biologics group (p>0.05) [Figure 2].
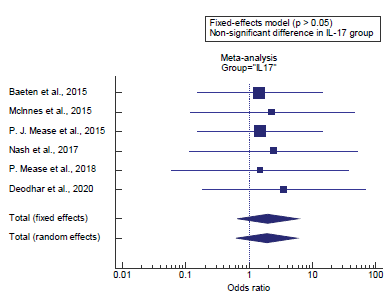
Concerning the secondary outcome measures, we found 6 studies reported the incidence of malignancy in IL-17 users. I2 (inconsistency) was 0% with a non-significant Q test for heterogeneity (p>0.05), so fixed-effects model was carried out; with overall OR= 1.97 (95% CI=0.661 to 5.91).
Using the fixed-effects model, the meta-analysis process revealed a non-significant difference in the incidence of malignancy in IL-17 users in the Biologics group compared to the Non-biologics group (p>0.05) [Figure 3].
Discussion
This work aims to determine the incidence of malignancies in spondyloarthritis (SpA) patients treated with biologics. Regarding patients’ characteristics, the total number of patients in all the included studies was 4395 patients, with 2878 patients in the Biologics group, and 1517 patients in the Non-biologics group, while their average follow-up time was (12 months).
Concerning the primary outcome measure, we found 11 studies reported an overall incidence of malignancy with a total number of patients (N=4395).
Using the fixed-effects model, the meta-analysis process revealed a non-significant difference in the overall incidence of malignancy in the Biologics group compared to the Nonbiologics group (p>0.05), which came in agreement with Kwan et al., [17] van der Heijde et al. [18] and Ruderman. [19]
Kwan et al. reported that, the hazard of malignancy with biologics in SpA, overall, as well as by using classes of biologics in every SpA kind. All pooled ORs had wide (p>0.05) except IL- 17 inhibitors in peripheral SpA compared with non-biologics, which had (p<0.05) when pooled using Peto’s method, but not when pooled using the MH method with TACC. Overall, the pooled OR was 1.42. For most comparisons of biologics with non-biologic, the ORs had wide 95% CI with (p>0.05) (no statistically significant), except for IL-17 inhibitors in peripheral SpA in comparison with non-biologic, specifically, the pooled ORs were 2.77 for IL-17 inhibitors in peripheral SpA, 2.30 TNF-A inhibitors in axial SpA, 1.81 for IL-17 inhibitors in axial SpA. [17]
Van der Heijde et al. reported that injection site reactions were reported in 4 (5%) of 86 patients inside the placebo group, (13%) of patients within the Ixekizumab group, (4%) of patients in the ixekizumab group, and (8%) of sufferers within the adalimumab group. One extreme injection site response becomes reported within the ixekizumab group; all other injection site reactions were slight or moderate in severity. Patients in the ixekizumab group (inclusive of the affected person with an excessive reaction) and one affected person within the adalimumab group discontinued treatment due to injection site reactions. No malignancies were reported in any treatment group. [18]
Ruderman reported that, overall, the safety of biologic and non-biologic DMARDs is quite affordable, mainly in mild of the efficacy of these agents. Careful monitoring through physicians familiar with the agents used is warranted, at the side of screening wherein indicated. While a few hazard factors for toxicity are clear (persistent or recurrent infections), others are more controversial (chronic obstructive pulmonary disorder as a danger for pulmonary infection with abatacept), so that clinical judgment can also be necessary. [19]
Concerning the secondary outcome measures, we found 5 studies reported the incidence of malignancy in TNF-A users. Using the fixed-effects model, the meta-analysis process revealed a non-significant difference in the incidence of malignancy in TNF-A users in the Biologics group compared to the Non-biologics group (p>0.05). Which came in agreement with Hernández et al., [20] Hellgren et al., [21] Kwan et al., [17] Favalli et al. [22] and Ruderman. [19]
Hernández et al. reported a, decrease prevalence of CAE in AS and PsA sufferers in comparison with RA sufferers. Reports describe an increase in cutaneous lesions and skin malignancies in RA patients and an increase in the rate of infections, which include cutaneous infections and viral infections, particularly herpes virus. The reason for this is not clear. However, RA patients are exposed to glucocorticoids more regularly than AS and PsA patients and glucocorticoids are related to cutaneous lesions. Various researches have suggested an association between using leflunomide and pores and skin lesions, especially in patients who acquired leflunomide in combination with TNF antagonists. [20]
Hellgren et al. reported that, for the duration of 185 236 personyears within the TNFi-naïve SpA cohort, 1188 incident cancers occurred (crude incidences 598 per one hundred individual/ year). Compared with the general population cohort (5153 cancers throughout 862 480 people/year) (crude incidence 652 per 100 000 people/year) this corresponded to an RR of most cancers overall in TNFi-naïve SpA of 1.1. This chance remained in large part the same when they estimated the risk of most cancers normal in TNFi-naïve AS, PsA, and SpA. There were no considerable differences in cancer hazard overall in TNFi-naïve male patients with SpA (RR=1.1) and female patients (RR=1.0) as compared with their general population comparator subjects. They made the following key observations: (i) there had been no increased dangers of cancer overall, nor of the six most common cancer types in TNFi-treated patients as compared with TNFinaïve patients with SpA, (ii) there has been a decreased chance of colorectal cancer in TNFi-naïve patients with AS as opposed to the general population. [21]
Kwan et al. reported that meta-analysis found an OR point estimate<1 (OR 0.98) with TNF-A in AS (a specific subtype of axial SpA), but it included only three RCTs (versus six RCTs in their study), one of which only had a 12-week follow-up. A 10- year registry-based study, meanwhile, found no elevated risk of overall malignancy (relative risk, 0.90) for TNF-A inhibitors in PsA, compared with TNF-A-naïve patients. [17]
Favalli et al. reported that specific AEs such as infections had been proven to be more frequent within the first levels of TNFi treatment, with a subsequent decrease for longer treatments. However, their data are very reassuring in demonstrating the long-term favorable TNFi safety profile for the treatment of SpA since the discontinuation rate due to AEs in their cohort did not increase over time, with a very low long-time period prevalence of more serious AEs such as malignancies and cardiovascular events. Of note, they discovered no large difference within the lengthy-time period safety profile of individual anti-TNF dealers, showing a similar pattern of withdrawal because of AE. [22] Ruderman reported that meta-analyses of multiple clinical trials, however, have now not observed an expanded hazard of serious infection in patients treated with abatacept. Combining abatacept with other biologic treatment options is contraindicated because of much greater risk of serious infection certainly; combinations of any biologics should be prevented within the absence of additional data to guide this approach. Abatacept, unlike TNF inhibitors, has no longer been associated with an increased hazard of opportunistic infection. Certainly, abatacept did no longer exacerbate infection in a mouse model of continual TB. Data from clinical trials have no longer recommended that abatacept is related to an increased hazard of malignancy. [19] Our result came in disagreement with Atzeni et al. [23] and Westhovens et al. [24]
Atzeni et al. reported that, of the 2421 SpA patients (males, 52.2%; mean age 47 ± 13 years; median disease duration three years), 50 developed at least one of 56 malignancies during the follow-up period of up to 12 years of treatment with TNFi. The overall prevalence was 6.5/1000 patient/year of followup 7.3/1000 patient/year in those treated with ADA; 6.1/1000 patient/year in those treated with ETN; and 5.4/1000 patient/ year in those treated with INF while in the general population was 5.1/1000 patient/year. The incidence of malignancies among SpA patients treated with the three TNFi was higher than in the general population; having had a previous solid cancer is predictive of a new malignancy. [23]
Westhovens et al. reported that 231 patients with a mean age of 47.86 y were included. In his study population, (2.6%) developed a malignancy after the start of the anti-TNF remedy. the general prevalence rate of malignancy in their look at population is 5.3% according to one hundred thousand patients/ year, indicating a higher occurrence as compared to the Belgian population. They see a higher incidence rate in females as properly in men; standardized incidence ratios are inside the equal range for each gender. [24]
Concerning the secondary outcome measures, we found 6 studies reported the incidence of malignancy in IL-17 users.
Using the fixed-effects model, the meta-analysis process revealed a non-significant difference in the incidence of malignancy in IL-17 users in the Biologics group compared to the Non-biologics group (p>0.05), which came in disagreement with Kwan et al. [17]
Kwan et al. reported that the comparison of IL-17 inhibitors with no biologics in peripheral SpA was the only one that performed statistical importance. The statistical importance was best observed with Peto’s method, but now not the MH technique with TACC. This difference maybe because of the tendency of MH with TACC to ‘pull’ the ORs towards no effect (OR=1), resulting in greater conservative OR estimates and a 95% CI that crosses OR=1. Nevertheless, all 8 RCTs for IL-17 inhibitors in peripheral SpA examined completely patients. [17]
Conclusion
To conclude, we found no evidence for elevated risk of malignancy with the use of biologics in SpA. We were also unable to assess the long-term risk of biologics in SpA, which requires longer follow-up durations, and further investigatory studies.
Competing Interests
The authors declare that they have no competing interests. All the listed authors contributed significantly to the conception and design of study, acquisition, analysis, and interpretation of data and drafting of the manuscript, to justify authorship.
REFERENCES
- Cici D, Corrado A, Rotondo C, Cantatore FP. Wnt signaling and biological therapy in rheumatoid arthritis and spondyloarthritis. Int J Mol Sci. 2019;20:5552.
- Caso F, Costa L, Del Puente A, Di Minno MND, Lupoli G, Scarpa R, et al. Pharmacological treatment of spondyloarthritis: exploring the effectiveness of nonsteroidal anti-inflammatory drugs, traditional disease-modifying antirheumatic drugs and biological therapies. Ther Adv Chronic Dis. 2015;6:328-338.
- Poddubnyy D, Sieper J. What is the best treatment target in axial spondyloarthritis: tumour necrosis factor α, interleukin 17, or both? Rheumatology 2018;57:1145-1150.
- Deodhar A, Yu D. Switching tumor necrosis factor inhibitors in the treatment of axial spondyloarthritis. Semin Arthritis Rheum. 2017;47:343-350.
- Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Bmj 2009;339.
- Dijkmans B, Emery P, Hakala M, Leirisalo-Repo M, Mola EM, Paolozzi L, et al. Etanercept in the longterm treatment of patients with ankylosing spondylitis. J Rheumatol. 2009;36:1256-1264.
- Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor α antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four–week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum. 2009;60:976-986.
- Bao C, Huang F, Khan MA, Fei K, Wu Z, Han C, et al. Safety and efficacy of golimumab in Chinese patients with active ankylosing spondylitis: 1-year results of a multicentre, randomized, double-blind, placebo-controlled phase III trial. Rheumatology 2014;53:1654-1663.
- Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373:2534-2548.
- McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet 2015;386:1137-1146.
- Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, Van Der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373:1329-1339.
- Kavanaugh A, Husni ME, Harrison DD, Kim L, Lo KH, Leu JH, et al. Safety and efficacy of intravenous golimumab in patients with active psoriatic arthritis: results through week twenty-four of the GO-VIBRANT study. Arthritis Rheum. 2017;69:2151-2161.
- Nash P, Kirkham B, Okada M, Rahman P, Combe B, Burmester G-R, et al. Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. The Lancet. 2017;389:2317-2327.
- Landewé R, Sieper J, Mease P, Inman RD, Lambert RG, Deodhar A, et al. Efficacy and safety of continuing versus withdrawing adalimumab therapy in maintaining remission in patients with non-radiographic axial spondyloarthritis (ABILITY-3): a multicentre, randomised, double-blind study. The Lancet. 2018;392:134-144.
- Mease P, van der Heijde D, Landewé R, Mpofu S, Rahman P, Tahir H, et al. Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double-blind, phase III FUTURE 5 study. Ann Rheum Dis. 2018;77:890-897.
- Deodhar A, Blanco R, Dokoupilová E, Hall S, Kameda H, Kivitz AJ, et al. Secukinumab improves signs and symptoms of non-radiographic axial spondyloarthritis: primary results of a randomized controlled phase III study. Arthritis Rheum. 2020.
- Kwan YH, Lim KK, Fong W, Goh H, Ng L, Haaland B, et al. Risk of malignancies in patients with spondyloarthritis treated with biologics compared with those treated with non-biologics: a systematic review and meta-analysis. Ther Adv Musculoskelet Dis. 2020;12:1759720X20925696.
- van der Heijde D, Wei JC-C, Dougados M, Mease P, Deodhar A, Maksymowych WP, et al. Ixekizumab, an interleukin-17A antagonist in the treatment of ankylosing spondylitis or radiographic axial spondyloarthritis in patients previously untreated with biological disease-modifying anti-rheumatic drugs (COAST-V): 16 week results of a phase 3 randomised, double-blind, active-controlled and placebo-controlled trial. The Lancet. 2018;392:2441-2451.
- Ruderman EM. Overview of safety of non-biologic and biologic DMARDs. Rheumatology. 2012;51:vi37-vi43.
- Hernández MV, Sanmartí R, Cañete JD, Descalzo MA, Alsina M, Carmona L, et al. Cutaneous adverse events during treatment of chronic inflammatory rheumatic conditions with tumor necrosis factor antagonists: study using the Spanish registry of adverse events of biological therapies in rheumatic diseases. Arthritis Care Res. 2013;65:2024-2031.
- Hellgren K, Dreyer L, Arkema EV, Glintborg B, Jacobsson LT, Kristensen L-E, et al. Cancer risk in patients with spondyloarthritis treated with TNF inhibitors: a collaborative study from the ARTIS and DANBIO registers. Ann Rheum Dis. 2017;76:105-111.
- Favalli EG, Selmi C, Becciolini A, Biggioggero M, Ariani A, Santilli D, et al. Eight-year retention rate of first-line tumor necrosis factor inhibitors in spondyloarthritis: a multicenter retrospective analysis. Arthritis Care Res. 2017;69:867-874.
- Atzeni F, Carletto A, Foti R, Sebastiani M, Panetta V, Salaffi F, et al. Incidence of cancer in patients with spondyloarthritis treated with anti-TNF drugs. Joint Bone Spine. 2018;85:455-459.
- Westhovens I, Lories RJ, Westhovens R, Verschueren P, De Vlam K. Anti-TNF therapy and malignancy in spondyloarthritis in the Leuven spondyloarthritis biologics cohort (BIOSPAR). Clin Exp Rheumatol. 2014;32:71-76.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.