Interactions of Rosiglitazone and Anti‑Arrhythmic Drugs in Animal Model
- *Corresponding Author:
- Mr. Yousuf Mohiuddin Mohammed,
Department of Pharmacy, Jawaharlal Nehru Technology University, Hyderabad, India.
E-mail: yousufmohiuddin@gmail.com
Citation: Mohammed YM, Mohammed EI, Mohiuddin N, Syeda SS. Interactions of rosiglitazone and anti-arrhythmic drugs in animal model. Ann Med Health Sci Res 2012;2:152-6.
Abstract
Background: Diabetes increases the risk of vascular problems by two times compared with a healthy individual, with deposition of fats in blood vessel and this includes cardiovascular disease. The treatment regimens for patients suffering from both diseases generally include prolonged use of anti‑diabetic drugs for diabetes and anti‑arrhythmic drugs for cardiac arrhythmias. Aim: The aim of the study is to compare the influence of Mexiletine and Disopyramide on the pharmacodynamics (PDs) of Rosiglitazone in normal and diabetic rats. Materials and Methods: The study was conducted in normal rats and diabetic induced rats (with Alloxan monohydrate 100 mg/kg body weight). Albino rats weighing between 160 and 280 g were administered oral doses of Rosiglitazone 0.72 mg/kg, Mexiletine 36 mg/kg, or Disopyramide 18 mg/kg of bodyweight and their combination, with 1 week of washout between treatments. Eighteen rats were divided into three sub‑sets with six rats in each sub‑set. After 4 days, the blood glucose was estimated to confirm the diabetes. The Analysis of Covariance (ANCOVA) using MedCalc® software Version 11.6.1.0 was performed to analyze mean change in blood glucose between treatments with body weight as co‑variable and treatment as factor for normal and diabetic rats. Results: No statistically significant difference in mean change in blood glucose between Rosiglitazone in comparison with Rosiglitazone + Mexiletine or Rosiglitazone + Disopyramide was observed in normal and diabetic rats (P = 0.606). The maximum mean change in blood glucose for Rosiglitazone and Rosiglitazone + Mexiletine or Rosiglitazone + Disopyramide was observed at 1 h and 8 h in normal and diabetic rats. The post hoc analysis showed baseline correction method has increased the reliability of the results (P < 0.001). Conclusion: The study concludes that PD activity of Rosiglitazone was not affected by the anti‑arrhythmic drugs. This study introduced a new statistical methodology for analyzing the blood glucose endpoint.
Keywords
Diabetes, Disopyramide, Mexiletine, Pharmacodynamics, Thiazolidinediones
Introduction
Diabetes mellitus is one of the chronic metabolic disorders characterized by the high blood sugar (glucose) levels, which results from defects in insulin secretion and/or.[1] The classical symptoms of diabetes are polyuria, polydipsia, and polyphagia. The symptoms may develop rapidly in type 1 diabetes while they develop much more slowly in type 2 diabetes.[2] Diabetes increases the risk of vascular problems by two times and this includes cardiovascular disease. The prevalence of diabetes in India is currently 1-5% (0.06/1.12 billion) and there are about 143 million people worldwide suffering from diabetes.[1,2]
The treatment regimens for patients generally include prolonged use of anti‑diabetic drugs for diabetic patients and anti‑arrhythmic drugs for cardiac arrhythmias. Polypharmacy assumes importance in present clinical practice since newer molecules are introduced and clinicians face newer challenges in managing multiple diseases and treatment regimens. The clinical observations and patient symptoms are essential in noting the pharmacodynamic (PD) interactions of drugs. To understand the mechanism of interactions and confirm the presence of any such interaction, the animal models are widely used. In essence, animal model studies help us in understanding the underlying mechanisms in both healthy and disease‑induced animal models. This coupled with few human clinical studies could help in developing a rational drug therapy with optimum benefits in humans.[3‑5]
Rosiglitazone belongs to a class of Thiozolidinedione and is commonly prescribed in combination with sodium channel blockers like Mexiletine or Disopyramide (anti‑arrthymic drugs). The aim of the study was to compare the influence of Mexiletine and Disopyramide on the pharmacodynamics (PDs) of Rosiglitazone in normal and diabetic rats to identify the presence of PD interaction in animal model.
Materials and Methods
The albino rats of either sex, weighing between 160 and 280 g were procured from Sainath Agencies, Hyderabad, India. The animals were maintained under standard laboratory conditions at ambient temperature of 25 ± 2°C with 12 h light/12 h dark cycle. They were fed with standard pellet diet and water.
As per the standard requirements, these animals were fasted for 12 h before the experiment, and during the experiment they were withdrawn from food and water. The prior approval for conducting the experiments in rats was obtained from Sultan‑Ul‑loom College of Pharmacy (SUCP) ‑ Institutional Animal Ethics Committee, Hyderabad, India. The laboratory used for carrying out the experiment was approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, Reg. No. [IAEC/SUCP/05/2010]. The experiments were conducted according to guidelines provided by CPCSEA.
The blood glucose levels were estimated by Glucose Oxidase method using the SPAN Diagnostic Glucose Kit, Surat, India. This method is also called as the glucose oxidase and perioxidase (GOD/POD) method.[6]
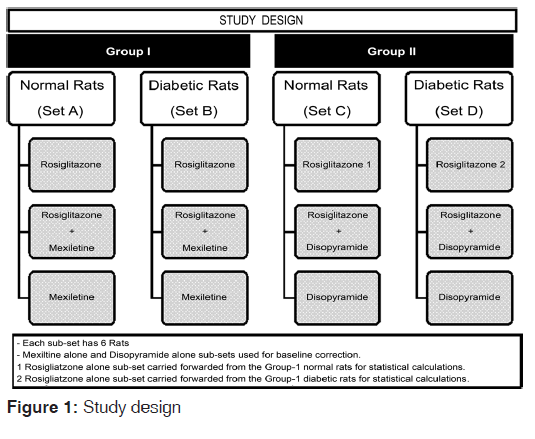
The study design is represented in the Figure 1. The rats were divided into four sets for ease of statistical analyses purpose.
The dose of Rosiglitazone orally administered was 0.72 mg/kg body weight. The dose of Mexiletine orally administered was 36 mg/kg bodyweight and Disopyramide dose orally administered was 18 mg/kg bodyweight, dose calculated based on human dose. [7‑9] A 1‑week washout period was provided in between treatments. The Rosiglitazone was administered 30 minutes after Mexiletine or Disopyramide when used in combination.
To induce diabetes in rats, an intra‑peritoneal administration of Alloxan monohydrate 100 mg/kg body weight was performed. After 4 days, the blood glucose was estimated to confirm the diabetes. Later, an additional dose of Alloxan 50 mg/kg body weight was intra‑peritoneally administered if increase in blood glucose was not seen.[10] The blood glucose levels of 200 mg/ dL and above was required to classify the rats as diabetic, and such rats were included in the study. The blood glucose was estimated by collecting blood samples from retro‑orbital plexus at intervals of 0, 1, 2, 3, 4, 6, 8, 10, and 12 h by GOD/POD method for both normal and diabetic rats.
The statistical comparison was performed between treatments in each of the sets described in Figure 1. The baseline correction method for Sets A and B was performed by subtracting the observed mean blood glucose values for Mexiletine group at each time point (i.e. 1 h and so on) from individual rats from the two treatment groups. This resulted in mean change in blood glucose at each time point for these groups. The rationale for the baseline correction was that Mexiletine alone is not expected to show any effect on blood glucose, and this method also accounted for intraday variation of blood glucose.
Similarly, in the other two sets (Sets C and D), Disopyramide was used for baseline correction. An analysis of covariance (ANCOVA) using MedCalc® software Version 11.6.1.0 was performed on change in blood glucose values between these groups. As different weights of rats were involved in the experiment, this was accounted in the statistical model as co‑variable and treatment as factor. The Bonferroni correction was included as part of the model in MedCalc® software. A P value of < 0.05 was considered to be statistically significant difference between groups. It was planned to statistically analyze two time points with maximum change in blood glucose for each set. The rationale was to minimize the use of numerous statistical tests and it was expected that difference between treatments can be determined at maximum change in blood glucose. The observed blood glucose values and change in blood glucose for each time point were presented.
Results
The mean and standard deviation (SD) of blood glucose level and mean change in blood glucose level at each time point for normal rats is presented in the Table 1. The baseline correction method was used at each time point as defined earlier in the method section. The maximum amount of change in blood glucose was observed at first and eighth hour in both the sets of comparison and this may be due to biphasic action of Rosiglitazone [Table 1].
| Hour | Rosiglitazone | Rosiglitazone+Mexiletine | Rosiglitazone | Rosiglitazone+Mexiletine |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| N=6 | N=6 | N=6 | N=6 | |
| Observed blood glucose values | Change from baseline in observed blood glucose values1 | |||
| 0 | 91.7 (3.38) | 93.5(3.5) | -4.1(3.3) | -2.3(3.5) |
| 1 | 44.3 (6.36) | 44.6 (6) | -31.5(10.7) | -33.3 (6) |
| 2 | 61.5 (9.45) | 49.16 (3.6) | -5.3(7.5) | -17.6(3.7) |
| 3 | 65.7 (10.1) | 60.6 (3.96) | -8.3 (10.2) | -13.3(3.9) |
| 4 | 75.7 (3.0) | 74.3 (2.93) | 10.8(3.0) | 7.03(7.7) |
| 6 | 63.7 (10.5) | 50.8 (4.16) | -0.33(10.5) | -13.1(4.1) |
| 8 | 50.8 (3.96) | 45.8 (4.36) | -30.8 (3.9) | -35.8(4.3) |
| 10 | 80.8 (2.2) | 81.16 (3.0) | -7.6(2.2) | -7.3(3.0) |
| 12 | 86.8 (3.30) | 89.16 (3.1) | -5.6(3.3) | -4.8(3.7) |
| Set C: Comparison of Rosiglitazone alone treatment and Rosiglitazone+Disopyramide treatment | ||||
| Hour | Rosiglitazone | Rosiglitazone+Disopyramide | Rosiglitazone | Rosiglitazone+Disopyramide |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| N=6 | N=6 | N=6 | N=6 | |
| Observed blood glucose values | Change from baseline in observed blood glucose values2 | |||
| 0 | 91.7 (3.38) | 96.16(3.77) | -3.5(3.3) | 0.9 (3.7) |
| 1 | 44.3 (6.36) | 46.8(9.9) | -28.9 (6.3) | -26.4 (5) |
| 2 | 61.5 (9.45) | 53.16(7.29) | -2.7(7.5) | -11(7) |
| 3 | 65.7 (10.1) | 68 (6.05) | 1.1 (10.2) | -3.5 (6) |
| 4 | 75.7 (3.0) | 60.3 (7.59) | -7.9 (3) | -7.3(7.6) |
| 6 | 63.7 (10.5) | 52.6 (6.41) | -6.6 (10.5) | -4.3(6.4) |
| 8 | 50.8 (3.96) | 47.5(5.9) | -26.4 (3.9) | -29.8(5.9) |
| 10 | 80.8 (2.2) | 80.16(4.77) | -7.4(2.2) | -8(4) |
| 12 | 86.8 (3.30) | 90.83(5.11) | -3.1(3.3) | 0.8 (5) |
Rosi: Rosiglitazone, SD: Standard deviation, 1Mean blood glucose values of Mexiletine were used for baseline correction at each time point, 2Mean blood glucose values of Disopyramide were used for baseline correction at each time point
Table 1: Observed mean blood glucose and mean change in blood glucose values (mg/dL) in normal rats at each time point.
As the maximum amount of change in blood glucose was seen at first and eighth hour, therefore as planned at these time points the analysis for statistical significance was performed. The statistical analysis was performed using ANCOVA in MedCalc® software with treatment as factor and weight as co‑variable in normal rats. The resulted showed that at 1 and 8 hour, there was no statistically significant difference in mean change in blood glucose between the treatments (P > 0.606) in the normal rats. Therefore, there is no change in glucose effect of Rosiglitazone with or without Mexiletine and Disopyramide in normal rats.
The mean and SD of blood glucose levels and mean change in blood glucose levels at each time point for diabetic rats is presented in the Table 2. Further statistical analysis did not show any significant difference between treatments, thereby indicating no change in glucose effect of Rosiglitazone with or without Mexiletine and Disopyramide in Diabetic rats.
| Hour | Rosiglitazone | Rosiglitazone+Mexiletine | Rosiglitazone | Rosiglitazone+Mexiletine |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| N=6 | N=6 | N=6 | N=6 | |
| Observed blood glucose values | Change from baseline in observed blood glucose values1 | |||
| 0 | 288.16 (15.6) | 289.8 (24.1) | 4 (38) | 4.6 (23) |
| 1 | 135.3 (17.7) | 134.6 (12.6) | -89 (17) | -90(12) |
| 2 | 153.6 (22.7) | 155.6 (11.7) | -48 (22) | -46(11) |
| 3 | 198.3 (26) | 185.8 (14.9) | -1.6 (26) | -14(14) |
| 4 | 216 (24.5) | 224.6 (25.9) | 6.3 (24) | 14 (25) |
| 6 | 194 (21.9) | 147.6 (10.3) | 8.1 (25) | -38(10) |
| 8 | 148.3 (16.75) | 141.6 (12.5) | -82 (16) | -89(12) |
| 10 | 229.1 (27.7) | 224.8 (16.4) | -18 (27) | -23(16) |
| 12 | 249 (26.3) | 261.6 (21.9) | -14 (26) | -2.3 (21) |
| Set D: Comparison of Rosiglitazone alone treatment and Rosiglitazone+Disopyramide treatment | ||||
| Hour | Rosiglitazone | Rosiglitazone+Disopyramide | Rosiglitazone | Rosiglitazone+Disopyramide |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| N=6 | N=6 | N=6 | N=6 | |
| Observed Blood Glucose values | Change from baseline in observed blood glucose values2 | |||
| 0 | 288.16 (15.6) | 288.5 (19.91) | 6 (38) | 6.5 (19) |
| 1 | 135.3 (17.7) | 135.6 (10.7) | -81 (17) | -84(14) |
| 2 | 153.6 (22.7) | 154.1 (9.55) | -42 (22) | -41 (9) |
| 3 | 198.3 (26) | 210.5 (16.9) | 3.3 (26) | 13 (19) |
| 4 | 216 (24.5) | 183.6 (7.7) | 23.3 (24) | -9.3(7.6) |
| 6 | 194 (21.9) | 152.3 (10.14) | 23.1 (25) | -18(10) |
| 8 | 148.3 (16.75) | 140.6 (9.9) | -81 (16) | -89 (9) |
| 10 | 229.1 (27.7) | 217.5 (15.52) | -15 (27) | -27.5 (15) |
| 12 | 249 (26.3) | 257.6 (15.77) | -15.8 (26) | -7.3 (15) |
Rosi: Rosiglitazone, SD: Standard deviation, 1Mean blood glucose values of Mexiletine were used for baseline correction at each time point, 2Mean blood glucose values of Disopyramide were used for baseline correction at each time point
Table 2: Observed mean blood glucose and mean change in blood glucose values (mg/dL) in diabetes rats at each time point
A post hoc analysis of observed blood glucose levels at 1 h and 8 h using an ANCOVA model was performed for Mexiletine or Disopyramide alone treatments in comparison with Rosiglitazone and Rosiglitazone + Mexiletine or Disopyramide in normal and diabetic rats. The rationale was to identify whether Mexiletine or Disopyramide alone groups had any decrease in blood glucose level, thereby inducing bias in the baseline correction method.
The results showed a statistically significantly higher blood glucose in Mexiletine or Disopyramide alone groups in comparison with Rosiglitazone and Rosiglitazone + Mexiletine or Disopyramide in normal and diabetic rats (P < 0.001), thereby indicating that baseline correction method was not affected. An additional post hoc analysis showed no statistically significant difference in the observed blood glucose values at 1 h and 8 h using ANCOVA model for comparisons between Rosiglitazone and Rosiglitazone + Mexiletine or Disopyramide in normal and diabetic rats (P > 0.606).
Discussion
This study was an attempt to identify the PD interactions in animal model between anti‑arrhythmic drugs and Thiozolidinediones (Rosiglitazone) with a statistical methodology standards similar to the one used in human clinical trials.[11,12]
The Disopyramide drug is metabolized by hepatic P450 CYP3A4 Isozymes (Product information Norpace) and Rosiglitazone is also metabolized by the same enzyme. In theory, the PD interaction is due to inhibition of metabolism of Rosiglitazone and thereby increasing pharmacokinetic concentration, which may lead to increased insulin release. Additionally, Disopyramide is believed to have added PD effect with respect to insulin release.[13] Mexiletine is not metabolized by the same enzyme as of Rosiglitazone but is believed to increase insulin release.[14,15] Rosiglitazone produces hypoglycemic action by way of release of insulin from b cells of pancreas and also by increase in glucose uptake at cellular level.[16]
The normal rats served to analyze the hypoglycemic effect and diabetic rats served the purpose of analyzing the anti‑hyperglycemic effect. The maximum decrease in blood glucose values were observed at 1 h and 8 h with Rosiglitazone alone and in combination with Mexiletine and Disopyramide. This biphasic response in rats (interval of 7 h) may be due to its enterohepatic circulation.
There was no statistically significant difference between Rosiglitazone alone and in combination with Mexiletine or Disopyramide in normal and diabetic rats. However, several prior studies [17‑21] have showed a statistically significant difference between anti‑diabetic drugs in comparison with similar type of PD interaction‑producing drugs. The reason could vary, but importantly, the statistical methodology was not in accordance with the current study and general clinical standards.
The authors working earlier on blood glucose as an endpoint had several drawbacks in their statistical methodology,[17‑21] and this includes:
• The use of percentage for reduction of blood glucose for paired t‑test.
• The use of time point ‑ 0 h as baseline for comparison with all time points.
• The use of paired t‑test on percentages.
• Use of numerous paired t‑test without Bonferroni correction.
• Not accounting for body weight of animals used and intra‑day variation in blood glucose level.
The current study used ANCOVA model with body weights as co‑variable and treatment as factor. The baseline correction method at each time point has accounted for intra‑day variation drawbacks. However, the current study had a limitation of using Mexiletine or Disopyramide alone groups as baseline. Therefore, this was also analyzed further by post hoc analysis, and it was observed that these drugs alone did not have a statistically significant decrease in blood glucose.
Conclusion
The study concluded that PD activity of Rosiglitazone in rats was not affected by the anti‑arrhythmic drugs. The combination of anti‑arrhythmic drug with anti‑diabetics was found to be safe. This study introduced a new statistically methodology for analyzing the blood glucose endpoint and that could be used in future relevant studies.
Acknowledgements
Abdul Rehman, B. Pharm, Maimona Tahseen, B. Pharm, Saba Khatoon, B. Pharm, Raghu Ram, M. Pharm, Faculty, and support staff at Sultan‑ul‑loom College of Pharmacy.
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Brown JB, Pedula KL, Bakst AW. The progressive cost of complications in type 2 diabetes mellitus. Arch Intern Med 1999;159:1873-80.
- Bressler R, Johnson DG. Pharmacological regulation of blood glucose levels in non-insulin-dependent diabetes mellitus. Arch Intern Med 1997;157:836-48.
- Cerveny JD, Leder RD, Weart CW. Issues surrounding tight glycemic control in people with type 2 diabetes mellitus. Ann Pharmacother 1998;32:896-905.
- Manolis AS, Deering TF, Cameron J, Estes NA 3rd. Mexiletine: Pharmacology and therapeutic use. Clin Cardiol 1990;13:349-59.
- Siddoway LA, Woosley RL. Clinical pharmacokinetics of disopyramide. Clin Pharmacokinet 1986;11:214-22.
- Trinder P. Determination of blood glucose using an oxidaase-peroxidase system with a non-carcinogenic chemogen. J Clin Pathol 1969;22:158-61.
- Laurence DR, Bacharach AL. Evaluation of drug activities and pharmacometrics. Vol. 1. London and New York: Academic Press; 1964. p. 134-66.
- Mexiletine (Mexitil®) drug information. Rx list. Available from: http://www.rxlist.com/mexitil-drug.htm. [Last accessed on 2012 Jan 8].
- Disopyramide (Norpace®) drug information. Rx list. Available from: http://www.rxlist.com/norpace-drug.htm. [Last accessed on 2012 Jan 5].
- Heikkila RE. The prevention of alloxan-induced diabetes in mice by dimethyl sulfoxide. Eur J Pharmacol 1977;44:191-3.
- Diabetes mellitus: Developing drugs and therapeutic biologics for treatment and prevention. FDA. Available from: http://www . fda . gov/downloads/Drugs/ GuidanceComplianceRegulatoryInformation/Guidances/ ucm071624.pdf. [Last accessed on 2012 Jan 12].
- E9 Statistical Principles for Clinical Trials. Rx list. Available from: http://www.fda.gov/downloads/Drugs/ GuidanceComplianceRegulatoryInformation/Guidances/ UCM073137.pdf. [Last accessed on 2012 Jan 18].
- Goldberg IJ, Brown LK, Rayfield EJ. Disopyramide (Norpace) induced hypoglycemia. Am J Med 1980;69:463-6.
- Broly F, Vandamme N, Libersa C, Lhermitte M. The metabolism of mexiletine in relation to the debrisoquine/ sparteine-type polymorphism of drug oxidation. Br J Clin Pharmacol 1991;32:459-66.
- Chew CY, Collett J, Singh BN. Mexiletine: A review of its pharmacological properties and therapeutic efficacy in arrhythmias. Drugs 1979;17:161-81.
- Ferner RE, Chaplin S. The relationship between the pharmacokinetics and pharmacodynamics effects of oral hypoglycemic drugs . Clin Pharmacokinet 1987;12(Suppl 6):379-401.
- Mastan S, Kumar KE. Influence of non-nucleoside reverse transcriptase inhibitors (efavirenz and nevirapine) on the pharmacodynamic activity of gliclazide in animal models. Diabetol Metab Syndr 2009;1:15.
- Prashanth S, Kumar AA, Madhu B, Rama N, Sagar JV. Pharmacokinetic and pharmacodynamic drug interactions of carbamazepine and glibenclamide in healthy albino Wistar rats. J Pharmacol Pharmacother 2011;2:7-10.
- Manjunath S, Kugali SN, Deodurg PM. Effect of clonidine on blood glucose levels in euglycemic and alloxan-induced diabetic rats and its interaction with glibenclamide. Indian J Pharmacol 2009;41(Suppl 5):218-20.
- Satyanarayana S, Kumar KE, Rajasekhar J, Thomas L, Rajanna S,Rajanna B. Influence of aqueous extract of fenugreek-seed powder on the pharmacodynamics and pharmacokinetics of gliclazide in rats and rabbits. Therapy 2007;4:457-63.
- Mastan SK, Kumar KE. Influence of atazanavir on the pharmacodynamics and pharmacokinetics of gliclazide in animal models. Int J Diabetes Mellit 2010;2:56-60.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.