Is Glycated Haemoglobin an Alternative to Diagnose Diabetes Mellitus in a Northern Nigerian Population?
2 Department of Medicine, College of Health Sciences, University of Abuja, Abuja, Nigeria
3 Department of Medicine, Ahmadu Bello University, Zaria, Kaduna State, Nigeria
Citation: Lawal Y, et al. Is Glycated Haemoglobin an Alternative to Diagnose Diabetes Mellitus in a Northern Nigerian Population?. Ann Med Health Sci Res. 2018;8:98-102
This open-access article is distributed under the terms of the Creative Commons Attribution Non-Commercial License (CC BY-NC) (http://creativecommons.org/licenses/by-nc/4.0/), which permits reuse, distribution and reproduction of the article, provided that the original work is properly cited and the reuse is restricted to noncommercial purposes. For commercial reuse, contact reprints@pulsus.com
Abstract
Aim: To assess the use of glycated haemoglobin (HbA1c) as an alternative to diagnose diabetes mellitus in a Northern Nigerian population. Method and Results: Cluster sampling was done to select four hundred (400) subjects from ten (10) communities after informed consent from each participant. Demographic variables were recorded and obesity indices measured from each subject. Oral glucose tolerance test and HbA1c measurements were subsequently carried out. Though the HbA1c criteria missed some of the subjects diagnosed by the OGTT criteria, Receiver Operating Characteristics (ROC) curve showed that HbA1c level of > 6.5% was highly specific and sensitive in diagnosing diabetes with a discriminant ability of 91.3% and a high Youden index of 0.83. Conclusion: Glycated haemoglobin may be suitable to diagnose diabetes mellitus in some persons.
Keywords
Diabetes mellitus; Glycated haemoglobin; Glucose intolerance
Introduction
Diabetes mellitus (DM) is a state of chronic high plasma glucose levels due to impaired secretion and/ or action of insulin or both with resultant dysmetabolism of carbohydrate, lipids, and protein. [1] It affects 415 million persons, projected to reach 642 million in 2040 and a major cause of morbidity and mortality worldwide. [1,2]
Glycated hemoglobin (HbA1c) is formed by the non-enzymatic binding glucose to hemoglobin molecule. It is commonly used to monitor blood glucose control over the preceeding 2 to 3 months in diabetic patients. [3]
The American Diabetes Association (ADA) Standards of Medical Care call for the addition of the HbA1c test to diagnose diabetes and pre-diabetes as thus: HbA1c in the 5.7% to 6.4% range indicates pre-diabetes, and HbA1c ≥ 6.5% indicates diabetes. [3]
Furthermore, the American College of Physician (ACP) agrees that the use of HbA1c in addition to OGTT will increase the rate of diagnosis of pre-diabetes and DM and reduce the percentage of undiagnosed in the US. [4] This was supported by Jeon et al. [5] in their report which showed that FPG can underdiagnose glucose intolerance thus HbA1c should be acceptable as a complementary diagnostic test in Korean patients.
Oral glucose tolerance test (OGTT) remains the most popular test used in the diagnosis of glucose intolerance. This method is tedious, requires time, and involves more than one visit to the health centre. The discomfort to the clients also include being asked to fast overnight, and subsequently asked to ingest a glucose drink that causes nausea and abdominal discomfort. The need for a method that will ensure that the diagnosis of glucose intolerance is reached in one visit to a healthcare centre and devoid of the aforementioned discomforts to the clients has become necessary. This will encourage everyone especially high-risk individuals to routinely undergo diagnostic test for glucose intolerance. Hence, glucose intolerance can be diagnosed early, reducing morbidities and mortalities among the patients. We therefore assess the accuracy of diagnosing glucose intolerance using glycated hemoglobin and its relationship with some obesity indices in a Northern Nigerian population.
Materials and Methods
This was a cross-sectional observational study carried out in Zaria, Northern Nigeria which has a population of 975153 and located between latitude 112031” N and longitude 70 42” E. [6] Hausa-Fulani constitutes the main ethnic group with some settlers from other parts of the country.
Following approval from the Research Ethics committee of Ahmadu Bello University Teaching Hospital Zaria, four hundred (400) persons were enrolled from ten (10) communities (clusters). Simple random sampling was used to select forty (40) participants from each cluster and informed consent obtained from each of them.
Inclusion criteria included persons who were not known as initio to have diabetes mellitus while exclusion criteria included persons on glucose counter-regulatory medications (glucocorticoids, HIV protease inhibitors, thiazide diuretics etc), persons with diabetes mellitus and subjects who declined consent in whatever form.
Demographic details (age, sex, ethnic group, occupation, marital status) and history (medical, medication, social, and family history of diabetes, hypertension) were obtained for each subject. Obesity indices were measured with the help of a trained assistant.
Standing height was measured using a stadiometer (Seca 213 portable stadiometer, Seca North America, USA) with the subject’s head in the Frankfort horizontal plane (i.e., an imaginary line from the inferior border of the eye orbit to the ear canal is parallel to the floor and perpendicular to the vertical backboard. The height was then, measured to the nearest 0.1 cm. [7]
Subjects were asked to put on light wears and stand on the centre of a beam balance (Seca 700 series, Seca North America, USA) with weight distributed evenly and hands positioned at the sides. The weight was then, recorded in kilograms to the nearest 0.5 kg. [7] Each subject was asked to lower his/her underclothing to below the waist, put the hands on opposite shoulders, then the waist located along the mid-axillary line, mid-way between the costal margin and the iliac crest top. [7] Waist circumference was then, measured to the nearest 0.1cm using a measuring tape (NON 171330, 72”, Medline industries Inc., USA). [7] Furthermore, the hip circumference was measured to the nearest 0.1cm by placing the measuring tape around the hip at the greater trochanter level. If the greater trochanter was not palpable, the largest horizontal girth around the buttocks was used. [7]
In order to perform oral glucose tolerance test, subjects were asked to continue normal diet without restricting carbohydrate in their meals 72 hours prior to test. They were then instructed to fast for 8-12 hours overnight after which blood samples were drawn into fluoride oxalate bottles from their antecubital veins at 0900 hours for fasting plasma glucose levels.
Subjects were then given glucose drinks made up of 75g anhydrous glucose in 250 mls of water to ingest within 5 minutes. Two hours after the ingestion of glucose drinks, venous blood samples were drawn again. The plasma samples were separated by centrifugation for glucose determination using the glucose oxidase method via spectrophotometry.
For HbA1c determination, Clover HbA1c analyser (NGSP, CE, FDA certified and IFCC traceable) with boronate affinity binding precipitation and measuring method using spectrophotometry (wavelength 415 nm) was used. The analyser had a coefficient of variation of (3.5 to 4.0)%, measuring time 5 min, measuring temperature 17-32℃ and 4ul of heparin-anticoagulated venous blood sample was used.
The WHO criteria was used in the diagnosis of pre-diabetes (impaired fasting glucose [FPG 6.1 to 6.9 mmol/L], impaired glucose tolerance [2hrPPG 7.8 to 11.0 mmol/L]), and diabetes mellitus (FPG > 7.0 mmol/L and/ or 2hrPPG > 11.1 mmol/L). The American Diabetes Association (ADA) HbA1c criteria was used to diagnose pre-diabetes (HbA1c 5.7% to 6.4%) and diabetes (HbA1c > 6.5%).
Statistical Analysis
Data was entered into microsoft excel and analysed using SPSS 19 software by IBM SPSS inc. Continuous variables were compared using student ‘t’ test while categorical variables were compared using chi square. Results were recorded as means + standard deviation at 95% confidence interval. The relationship between plasma glucose levels and glycated hemoglobin, body mass index, waist circumference, and waist-hip ratio was tested using Pearson’s correlation. Receiver Operating Characteristics (ROC) curve was used to determine the sensitivity, specificity, and discriminant ability of HbA1c to diagnose glucose intolerance. P-value was taken as significant when less than or equal to 0.05.
Results
The use of HbA1c as an alternative to diagnose glucose intolerance in a northern Nigerian population was assessed in this study. Four hundred (400) subjects participated in the study with a male:female ratio of 1:1.1. As a result of incomplete data and laboratory findings, 4 participants were excluded from the analysis of data. Response rate was 99%.
The mean age (years) of subjects was 40.4±10.4 with mean BMI of 27.0±5.9 kg/m2. The mean waist circumference and waist-hip ratio were 87.9±12.3 cm and 0.93+0.16 respectively while the mean FPG and 2hrPPG were 5.17±2.19 mmol/L and 6.51±3.41mmol/L respectively. Glycated haemoglobin (HbA1c) mean value was 4.46% [Table 1].
| Variables | N | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|---|
| Age (yrs) | 396 | 18.00 | 69.00 | 40.44 | 10.39 |
| BMI (kg/m2) | 396 | 14.04 | 54.18 | 27.07 | 5.90 |
| WC (cm) | 396 | 63.00 | 127.50 | 87.91 | 12.28 |
| WHR | 396 | .52 | 1.64 | .93 | .16 |
| FPG (mmol/L) | 396 | 3.20 | 16.80 | 5.17 | 2.19 |
| 2HRPPG (mmol/L) | 396 | 3.40 | 25.40 | 6.51 | 3.41 |
| HbA1c (%) | 396 | 2.60 | 8.30 | 4.46 | .96 |
| Valid N (listwise) | 396 | - | - | - | - |
N: Number Of Subjects, SD: Standard Deviation, Yr: Years, BMI: Body Mass Index, WC: Waist Circumference, WHR: Waist-Hip Ratio, FPG: Fasting Plasma Glucose, 2HrPPG: Two-Hour Post-Prandial Plasma Glucose, HbA1c: Glycated Haemoglobin
Table 1: Showing the summary of clinical and laboratory variables of subjects.
The mean fasting plasma glucose (mmol/L) of male vs female subjects was 5.11±2.10 vs 5.22±2.27 while the mean 2-hour post 75g glucose load (mmol/L) was 6.42±3.18 vs 6.60±3.62 for male vs female subjects. Glycated haemoglobin (HbA1c) mean values (%) for male and female subjects were 4.49±0.94 and 4.54±0.97 respectively [Table 2].
| Variables | Female subjects | Male subjects | ||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| Age (yrs) | 207 | 41.26 | 11.17 | 189 | 39.54 | 9.41 |
| BMI (kg/m2) | 207 | 27.33 | 5.93 | 189 | 26.77 | 5.87 |
| WC (cm) | 207 | 88.09 | 11.63 | 189 | 87.72 | 12.98 |
| WHR | 207 | .90 | .15 | 189 | .97 | .16 |
| FPG (mmol/L) | 207 | 5.22 | 2.27 | 189 | 5.12 | 2.10 |
| 2HRPPG (mmol/L) | 207 | 6.60 | 3.62 | 189 | 6.42 | 3.18 |
| HbA1c (%) | 207 | 4.54 | .97 | 189 | 4.49 | .94 |
N: Number Of Subjects, SD: Standard Deviation, Yr: Years, BMI: Body Mass Index, WC: Waist Circumference, WHR: Waist-Hip Ratio, FPG: Fasting Plasma Glucose, 2HrPPG: Two-Hour Post-Prandial Plasma Glucose, HbA1c: Glycated Haemoglobin
Table 2: Showing the sex distribution of clinical and laboratory parameters of subjects.
The prevalence of glucose dysregulation using the OGTT criteria was 26.5% (pre-diabetes 17.2% and diabetes 9.3%). Female subjects with diabetes mellitus constituted 5.1% of all subjects while diabetic male subjects constituted 4.3% [Table 3].
|  Variables | Sex | Total | ||
|---|---|---|---|---|
| f | m | |||
| DM | Count | 20 | 17 | 37 |
| % within DM | 54.1% | 45.9% | 100.0% | |
| % within sex | 9.7% | 9.0% | 9.3% | |
| % of Total | 5.1% | 4.3% | 9.3% | |
| Normal | Count | 152 | 139 | 291 |
| % within normal | 52.2% | 47.8% | 100.0% | |
| % within sex | 73.4% | 73.5% | 73.5% | |
| % of Total | 38.4% | 35.1% | 73.5% | |
| pre-diabetes | Count | 35 | 33 | 68 |
| % within pre-diabetes | 51.5% | 48.5% | 100.0% | |
| % within sex | 16.9% | 17.5% | 17.2% | |
| % of Total | 8.8% | 8.3% | 17.2% | |
| Total | Count | 207 | 189 | 396 |
| % within sex | 100.0% | 100.0% | 100.00% | |
| % of Total | 52.3% | 47.7% | 100.00% | |
DM: Diabetes Mellitus, F: Female, M: Male
Table 3: Showing sex distribution of glucose intolerance by OGTT criteria.
Using the HbA1c criteria, the prevalence of glucose intolerance was 12.8% (pre-diabetes 9.3% and diabetes 3.5%). Female subjects with diabetes mellitus (HbA1c criteria) constituted 2.3% of all subjects while diabetic male subjects constituted 1.3% [Table 4].
| Variables | Sex | Total | ||
|---|---|---|---|---|
| f | m | |||
| DM | Count | 9 | 5 | 14 |
| % within DM | 64.3% | 35.7% | 100.0% | |
| % within sex | 4.3% | 2.6% | 3.5% | |
| % of Total | 2.3% | 1.3% | 3.5% | |
| Normal | Count | 185 | 160 | 345 |
| % within normal | 53.6% | 46.4% | 100.0% | |
| % within sex | 89.4% | 84.7% | 87.1% | |
| % of Total | 46.7% | 40.4% | 87.1% | |
| pre-diabetes | Count | 13 | 24 | 37 |
| % within pre-diabetes | 35.1% | 64.9% | 100.0% | |
| % within sex | 6.3% | 12.7% | 9.3% | |
| % of Total | 3.3% | 6.1% | 9.3% | |
| Total | Count | 207 | 189 | 396 |
| % within sex | 100.0% | 100.0% | 100.0% | |
| % of Total | 52.3% | 47.7% | 100.0% | |
DM: Diabetes Mellitus, F: Female, M: Male
Table 4: Showing sex distribution of glucose intolerance by HbA1c criteria.
Analysis of age-range quartiles showed that in the 50th percentile (2nd quartile age range), the prevalence of glucose intolerance (OGTT criteria) was 4.3% (pre-diabetes 2.3%, diabetes 2.0%) with the female and male subjects constituting 2.3% and 2.0% respectively [Table 5].
| Variables | Sex | Total | ||
|---|---|---|---|---|
| f | m | |||
| DM | Count | 4 | 4 | 8 |
| % within DM | 50.0% | 50.0% | 100.0% | |
| % within sex | 1.9% | 2.1% | 2.0% | |
| % of Total | 1.0% | 1.0% | 2.0% | |
| Normal | Count | 23 | 43 | 66 |
| % within normal | 34.8% | 65.2% | 100.0% | |
| % within sex | 11.1% | 22.8% | 16.7% | |
| % of Total | 5.8% | 10.9% | 16.7% | |
| pre-diabetes | Count | 5 | 4 | 9 |
| % within pre-diabetes | 55.6% | 44.4% | 100.0% | |
| % within sex | 2.4% | 2.1% | 2.3% | |
| % of Total | 1.3% | 1.0% | 2.3% | |
| Total | Count | 207 | 189 | 396 |
| % within sex | 100.0% | 100.0% | 100.0% | |
| % of Total | 52.3% | 47.7% | 100.0% | |
DM: Diabetes Mellitus, F: Female, M: Male
Table 5: Showing sex distribution of glucose intolerance (OGTT criteria) in the 50th percentile (2nd quartile) age range.
Analysis of the 50th percentile (2nd quartile age range) showed that the prevalence of glucose intolerance (HbA1c criteria) was 2.8% (prediabetes 2.3%, diabetes 0.5%) with the female and male subjects constituting 1.5% and 1.3% respectively [Table 6].
| Variables | Sex | Total | ||
|---|---|---|---|---|
| f | m | |||
| DM | Count | 2 | 0 | 2 |
| % within DM | 100.0% | .0% | 100.0% | |
| % within sex | 1.0% | .0% | .5% | |
| % of Total | .5% | .0% | .5% | |
| Normal | Count | 26 | 46 | 72 |
| % within normal | 36.1% | 63.9% | 100.0% | |
| % within sex | 12.6% | 24.3% | 18.2% | |
| % of Total | 6.6% | 11.6% | 18.2% | |
| pre-diabetes | Count | 4 | 5 | 9 |
| % within pre-diabetes | 44.4% | 55.6% | 100.0% | |
| % within sex | 1.9% | 2.6% | 2.3% | |
| % of Total | 1.0% | 1.3% | 2.3% | |
| Total | Count | 207 | 189 | 396 |
| % within sex | 100.0% | 100.0% | 100.0% | |
| % of Total | 52.3% | 47.7% | 100.0% | |
DM: Diabetes Mellitus, F: Female, M: Male
Table 6: Showing sex distribution of glucose intolerance (HbA1c criteria) in the 50th percentile (2nd quartile) age range.
There was a significant correlation between HbA1c and BMI (p<0.001), waist circumference (p=0.004), waist-hip ratio (p=0.003), FPG (p<0.001), and 2hrPPG (p<0.001) [Table 7].
| Variables | BMI | WC | WHR | FPG | 2hrPPG | HbA1c | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R | p | R | p | R | p | R | p | R | p | R | p | |
| BMI | 1 | 0.005 | 0.915 | 0.005 | 0.918 | 0.268 | <0.001 | 0.236 | <0.001 | 0.210 | <0.001 | |
| WC | 0.005 | 0.915 | 1 | 1.000 | <0.001 | 0.004 | 0.937 | 0.048 | 0.343 | 0.249 | 0.004 | |
| WHR | 0.005 | 0.918 | 1.000 | 0.001 | 1 | 0.003 | 0.950 | 0.047 | 0.352 | 0.259 | 0.003 | |
| FPG | 0.268 | <0.001 | 0.004 | 0.937 | 0.003 | 0.950 | 1 | 0.922 | <0.001 | 0.686 | <0.001 | |
| 2hrPPG | 0.236 | <0.001 | 0.048 | 0.343 | 0.047 | 0.352 | 0.922 | <0.001 | 1 | 0.670 | <0.001 | |
| HbA1c | 0.210 | <0.001 | 0.249 | 0.004 | 0.259 | 0.003 | 0.686 | <0.001 | 0.670 | <0.001 | 1 | |
BMI: Body Mass Index, WC: Waist Circumference, WHR:Waist-Hip Ratio, FPG: Fasting Plasma Glucose, 2hrppg: Two-Hour Post 75 G Glucose Load, HbA1c: Glycated Haemoglobin, R: Pearson’s Correlation, p:Significance Level
Table 7: Pearson’s correlation of obesity indices, plasma glucose, and HbA1c.
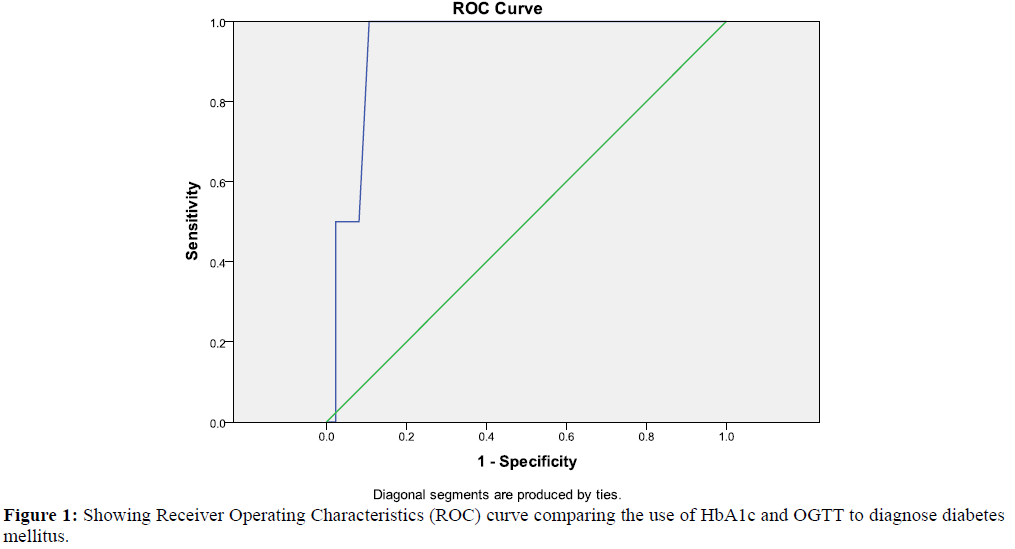
The Receiver Operating Characteristic (ROC) curve to assess the accuracy of HbA1c value of > 6.5% (ADA criteria) to diagnose diabetes mellitus showed that area under curve (AUC) was 94.2% (CI 88.8% - 99.6%), p=0.031. The sensitivity was 100% and the specificity was 82.6% with a discriminant ability of 91.3% and Youden index of 0.83 [Figure 1].
Discussion
The main aim of this study was to determine the accuracy of glycated haemoglobin (HbA1c) to diagnose glucose intolerance. The prevalence of diabetes by oral glucose tolerance test (OGTT) criteria was 9.3% while by HbA1c criteria was 3.5%. However age-range quartiles analysis showed the prevalence of pre-diabetes in the 2nd quartile to be 2.3% each by both the OGTT and HbA1c criteria. This shows that accuracy of diagnosis by HbA1c was best in the 2nd quartile (50th percentile) age-range in this study. In addition, HbA1c showed strong correlation with body mass index (BMI), waist circumference (WC), waist-hip ratio (WHR), fasting plasma glucose (FPG), and two-hour post 75 g glucose load (2hrPPG). This is in keeping with several studies including that of Jeon et al. [5] Nazaimoon et al. [8] and Bao et al. [9]
Sex distribution of glucose intolerance showed that the female subjects had higher prevalence of glucose intolerance by both the OGTT and HbA1c criteria. This can be explained by the higher BMI, waist circumference, and waist-hip ratio of the female subjects with their mean WC and WHR within abdominal obesity range for sex [Table 2]. Their higher obesity indices can further be explained by the mostly sedentary lifestyle of the women in these communities.
The Receiver Operating Characteristics (ROC) curve showed that HbA1c level of >6.5% was highly specific and sensitive in diagnosing diabetes with a discriminant ability of 91.3% and a high Youden index of 0.83. This is in tandem with the findings by Alqahtani et al. [10] Mehmet et al. [11] and Kharroubi et al. [12] in different studies.
However, initial analysis from our data showed that OGTT diagnosed more subjects with glucose intolerance than the HbA1c criteria. This is similar to reports by Darin et al. [13] and Fangjian et al. [14] where they demonstrated that HbA1c may miss diagnoses of pre-diabetes in some individuals. Darin et al. however, alluded to racial difference in the accuracy of HbA1c to diagnose pre-diabetes and diabetes. Genetic polymorphisms in the rate of formation and half-life of HbA1c among individuals and different races have been reported. [15] Furthermore, a recent large-scale study discovered a genetic variant of glucose-6 phosphate dehydrogenase (G6PD) found only in African Americans that may lead to shorter lifespan of red blood cells and thus false low measurements of HbA1c. [16]
Limitations of this study include the fact that conditions that can affect HbA1c levels like haemoglobinopathies, haemolytic diseases, etc. were not screened for. Lower-than-expected levels of HbA1c can be seen in people with shortened red blood cell lifespan, such as those with glucose-6-phosphate dehydrogenase deficiency, sickle-cell disease, or any other condition causing premature red blood cell death. [3,4] On the converse, higherthan- expected levels can be seen in people with a longer red blood cell lifespan such as vitamin B12 or folate deficiency, splenectomy, and aplastic anaemia. [3,4] However, the fact that apparently healthy adults with no known health condition were recruited for this study helped to mitigate the effect of this confounding factor. [17-23]
Conclusion
From our study, glycated haemoglobin may be suitable for the diagnosis of glucose intolerance especially in the 2nd quartile age range. Glycated haemoglobin can also be used as a complementary test to OGTT to increase the rate of diagnosis of glucose intolerance.
The possibility of racial difference in the accuracy of HbA1c to diagnose glucose intolerance should be explored by future research. Furthermore, large scale studies are recommended to assess for optimum HbA1c threshold for the diagnosis of diabetes and pre-diabetes in general and possibly in specific populations like Africans and African-Americans with certain genetic variant. More studies should also look at the influence of age-range quartiles in the accuracy of HbA1c to diagnose glucose intolerance.
Conflict of Interest
All authors disclose that there was no conflict of interest.
REFERENCES
- Baynes HW. Classification, pathophysiology, diagnosis, and management of diabetes mellitus. J Diabetes Metab 2015;6:541
- International Diabetes Federation. IDF Diabetes Atlas, 7th edn. Brussels, Belgium: International Diabetes Federation, 2015.
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2012;35.
- American College of Physicians. Trends in Prevalence and Control of Diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med 2014;160:517-525.
- Jeon JY, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, et al. Prevalence of diabetes and prediabetes according to fasting plasma glucose and HbA1c. Diabetes Metab J 2013;37:349-357
- The Geonames geographical database. Population of Zaria, Nigeria. Mongabay, 2012.
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey III; anthropometry procedures manual. Georgia, USA: Centers for Disease Control and Prevention 2016.
- Nazaimoon WMW, Isa SHM, Mohamad WBW, Khir AS, Kamaruddin NA, Kamarul IM, et al. Prevalence of diabetes in Malaysia and usefulness of HbA1c as a diagnostic criterion. Diabetic Medicine 2013;30:825-828.
- Bao Y, Ma X, Li H, Zhou M, Hu C, Wu H, et al. Glycated haemoglobin A1c for diagnosing diabetes in Chinese population: cross sectional epidemiological survey. Brit Med J 2010;340:2249.
- Alqahtani N, Khan WAG, Alhumaidi MH, Ahmed YAAR. Use of glycated hemoglobin in the diagnosis of diabetes mellitus and pre-diabetes and role of fasting plasma glucose, oral glucose tolerance test. Int J Prev Med 2013;4:1025-1029.
- Mehmet ON, Bahceci M, Aslan SL, Shawcross J, Harman E, Tutuncuoglu AP. Diagnosis of diabetes mellitus and pre-diabetes with fasting plasma glucose, oral glucose tolerance test and A1C level: A1C based screening may be a better diagnostic tool for diabetes mellitus. Med Science 2012;1:341-350.
- Kharroubi AT, Darwish HM, Abu Al-Halaweh AI, Khammash UM. Evaluation of glycated hemoglobin (HbA1c) for diagnosing type 2 diabetes and prediabetes among Palestinian Arab Population. PLoS ONE 2014;9:e88123.
- Darin EO, Mary KR, Herrick K, Ziemer DC, Twombly JG, Phillips LS. Screening for diabetes and pre-diabetes with proposed A1C-based diagnostic criteria. Diabetes Care 2010;33:2184-2189.
- Fangjian G, Douglas RM, Timothy GW. Use of HbA1c for diagnoses of diabetes and prediabetes: Comparison with diagnoses based on fasting and 2-Hr glucose values and effects of gender, race, and age. Metabolic Syndrome and Related Disorders 2014;12:258-268.
- Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, et al. Utility of hemoglobin A1c for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care 2011;34:1306-1311.
- Wheeler E, Leong A, Liu CT, Hivert MF, Strawbridge RJ, Podmore C, et al. Impact of common genetic determinants of haemoglobin A1c on type 2 diabetic risk and diagnosis in ancestrally diverse populations. A transethnic genome-wide meta-analysis. PLoS Med 2017;14:e1002383.
- Kapadia CR. Are the ADA hemoglobin A1c criteria relevant for the diagnosis of type 2 diabetes in youth? Curr Diab Rep 2013;13:51-55.
- Morris DH, Khunti K, Achana F, Srinivasan B, Gray LJ, Davies MJ et al. Progression rates from HbA1c 6.0–6.4% and other prediabetes definitions to type 2 diabetes: a meta-analysis. Diabetologia 2013;56:1489-1493.
- World Health Organisation. WHO STEPS instrument (version 3.0). Geneva: World Health Organisation 2014.
- Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 2014; 383:1068-1083.
- Kerner W, J. Brückel J. Definition, classification and diagnosis of diabetes mellitus. Exp Clin Endocrinol Diabetes 2014;122:384-386.
- Dall TM, Yang W, Halder P, Pang B, Massoudi M, Wintfeld N, et al. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 2014;37:3172-3179.
- Everlina MA, Wanda MA, Wim BB, Holleman F, Nierkens V, Barend JC, et al. Screening South Asians for type 2 diabetes and prediabetes: (1) comparing oral glucose tolerance and haemoglobin A1c test results and (2) comparing the two sets of metabolic profiles of individuals diagnosed with these two tests. BMC Endocrine Disorders 2013;13:8.




 The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.
The Annals of Medical and Health Sciences Research is a monthly multidisciplinary medical journal.